Kobayashi Lab/Research Center for Infectious Disease Control Department of Virology
The family Reoviridae comprises a group of nonenveloped viruses classified into 15 genera, and possessing 9–12 segmented double-stranded RNA genomes. This family includes important pathogens, including the rotaviruses, in humans and animals. In our laboratory, using original technology to generate recombinant Reoviridae viruses from cloned cDNAs, we are studying molecular mechanisms underlying Reoviridae virus replication and pathogenesis, and developing novel vaccine vectors.
1) Rotaviruses (RVs)
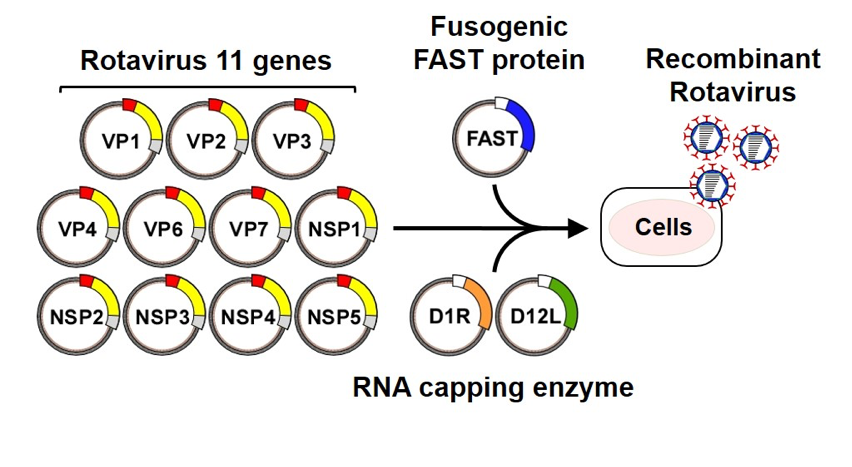
RVs are highly important pathogens that cause severe diarrhea in infants and young children worldwide. Understanding of the molecular mechanisms underlying the replication and pathogenesis of RVs has been hampered by the lack of a reverse genetics system that allows the synthesis of recombinant viruses from artificial genes. Recently, we developed a long-awaited plasmid-based reverse genetics system for RVs. This technique opens up new horizons for studying the replication and pathogenesis of RVs. We are investigating RV biology and developing vaccines and therapeutics using a combination of genetic, biochemical, and biophysical approaches.
2) Oncolytic viral therapy using reoviruses
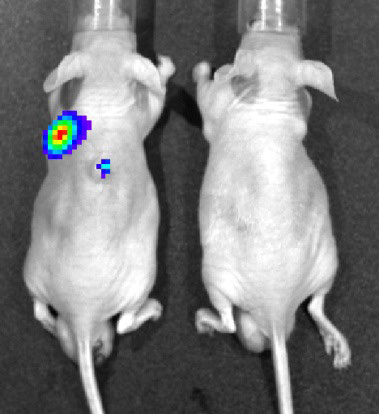
Mammalian orthoreoviruses (reoviruses) are members of the family Reoviridae and have a genome containing 10 segments of double-stranded (ds) RNA. Reoviruses are highly tractable experimental models for studying the replication and pathogenesis of dsRNA viruses. In the last decade, reoviruses have been evaluated as oncolytic agents against a variety of tumors, including head and neck, colon, breast, and pancreatic cancers, in animal models and humans. This is based on the observation that reoviruses induce cell death and apoptosis in tumor cells with an activated Ras signaling pathway. Wild-type reovirus-based oncolytic therapies are safe, but their efficacy is currently limited. We are developing safer and more effective reovirus-based cancer therapeutics by genetic modification.
3) Highly pathogenic bat-borne reovirus
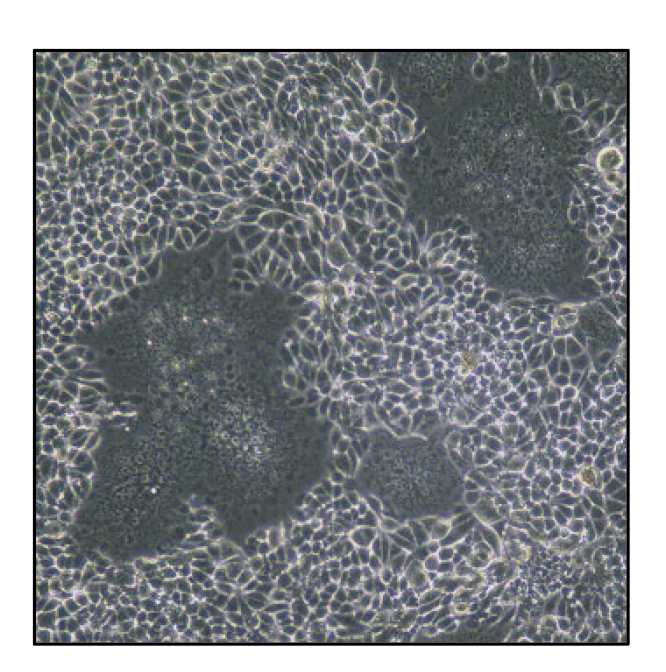
Nelson Bay reovirus (NBV) was isolated from a flying fox in 1968 but had not been associated with any disease. However, NBVs were recently isolated from human patients suffering from acute respiratory tract infections in Malaysia, Indonesia, China, and Japan. These isolates have given rise to increasing concerns about bat-transmitted reovirus infections in humans. We are investigating how NBV replicates and causes disease in vitro and in vivo.
-
Fig.1. Generation of recombinant rotaviruses from cloned cDNAs. BHK-T7 cell lines were transfected with the rotavirus 11 cDNA constructs and expression plasmids encoding FAST and vaccinia virus capping enzyme (D1R and D12L).
-
Fig. 2. Bioluminescence imaging of reovirus infection in human cancer xenografts. BALB/c nude mice transplanted with a human cancer cell line were infected intravenously with reporter reovirus.
-
Fig. 3.Nelson Bay orthoreovirus is capable of inducing cell–cell fusion in infected cells.
Staff
- Prof.: Takeshi Kobayashi
- Asst. Prof.: Tomohiro Kotaki
- Asst. Prof.: Takahiro Kawagishi
- Sa Asst. Prof.: Shohei Minami
Website
Publications
(1) Vero cell-adapted SARS-CoV-2 strain shows increased viral growth through furin-mediated efficient spike cleavage. Minami S. et al., Microbiology Spectrum (2023) e0285923
(2) Genetic engineering strategy for generating a stable dsRNA virus vector using a virus-like codon-modified transgene. Kanai Y. et al., Journal of Virology, (2023) 97(10):e0049223
(3) Characterization of Sialic Acid-Independent Simian Rotavirus Mutants in Viral Infection and Pathogenesis. Yamasaki M., et al., J. Virol. (2023) 97(1):e0139722
(4) N-Glycosylation of Rotavirus NSP4 Protein Affects Viral Replication and Pathogenesis. Nurdin JA., et al., J. Virol. (2023) 97(1):e0186122
(5) The nonstructural p17 protein of a fusogenic bat-borne reovirus regulates viral replication in virus species- and host-specific manners. Nouda R., et al., PLoS Pathog. (2022) 18(6):e1010553
(6) Development of an entirely plasmid-based reverse genetics system for 12-segmented double-stranded RNA viruses. Nouda R., et al., Proc Natl Acad Sci USA (2021) 118(42):e2105334118
(7) Generation of Genetically RGD σ1-Modified Oncolytic Reovirus That Enhances JAM-A-Independent Infection of Tumor Cells. Kawagishi T., et al. J Virol. (2020) Nov 9;94(23):e01703-20.
(8) Cell-cell fusion induced by reovirus FAST proteins enhances replication and pathogenicity of non-enveloped dsRNA viruses. Kanai Y., et al. PLoS Pathog. (2019) Apr 25;15(4):e1007675.
(9) Entirely plasmid-based reverse genetics system for rotaviruses. Kanai Y., et al., Proc Natl Acad Sci USA (2017) 114(9):2349-2354.
- Home
- Laboratories
- Kobayashi Lab