A novel toxin, inactivating heterotrimeric Gi GTPases, from Bordetella parapertussis (Horiguchi Lab, in PNAS)
Researchers (Dr. Yukihiro Hiramatsu and Prof. Yasuhiko Horiguchi) from Dept. of Molecular Bacteriology identified a novel toxin of B. parapertussis, which inactivates heterotrimeric Gi GTPases through demyristoylation of their α subunits and contributes to B. parapertussis-caused cough. The researchers named this toxin deacylating autotransporter toxin, DAT.
The Gram-negative bacteria B. pertussis and B. parapertussis cause pertussis (whooping cough) and pertussis-like disease, respectively. These diseases are characterized by severe paroxysmal coughing that persists for several weeks and imposes a significant burden on patients, especially infants. We previously presented a mouse-coughing model for pertussis cough and reported that pertussis toxin, which inactivates heterotrimeric GTPases of the Gi family through ADP-ribosylation of their α subunits, causes coughing in combination with Vag8 and lipid A (Hiramatsu et al., mBio. 13(2):e0319721, 2022). However, the mechanism of cough induced by B. parapertussis, which produces Vag8 and lipopolysaccharide (LPS) containing lipid A, but not pertussis toxin, remained to be elucidated. In this study, we demonstrated that a novel toxin named deacylating autotransporter toxin (DAT) of B. parapertussis inactivates heterotrimeric Gi GTPases through demyristoylation of their α subunits and contributes to cough production along with Vag8 and LPS, indicating that B. parapertussis utilizes DAT instead of pertussis toxin to cause paroxysmal coughing in hosts. In addition, immunization with DAT protected mice from coughing after B. parapertussis infection, while that with a current acellular pertussis vaccine did not. These results suggest that DAT has the potential as a vaccine antigen to prevent B. parapertussis-caused coughing.
This article was published in Proceedings of the National Academy of Sciences of the United States of America (PNAS) on Sep 26, 2023.
Title: “DAT, deacylating autotransporter toxin, from Bordetella parapertussis demyristoylates Gαi GTPases and contributes to cough”
Authors: Yukihiro Hiramatsu, Takashi Nishida, Natsuko Ota, Yuki Tamaki, Dendi Krisna Nugraha, Yasuhiko Horiguchi
Links
-
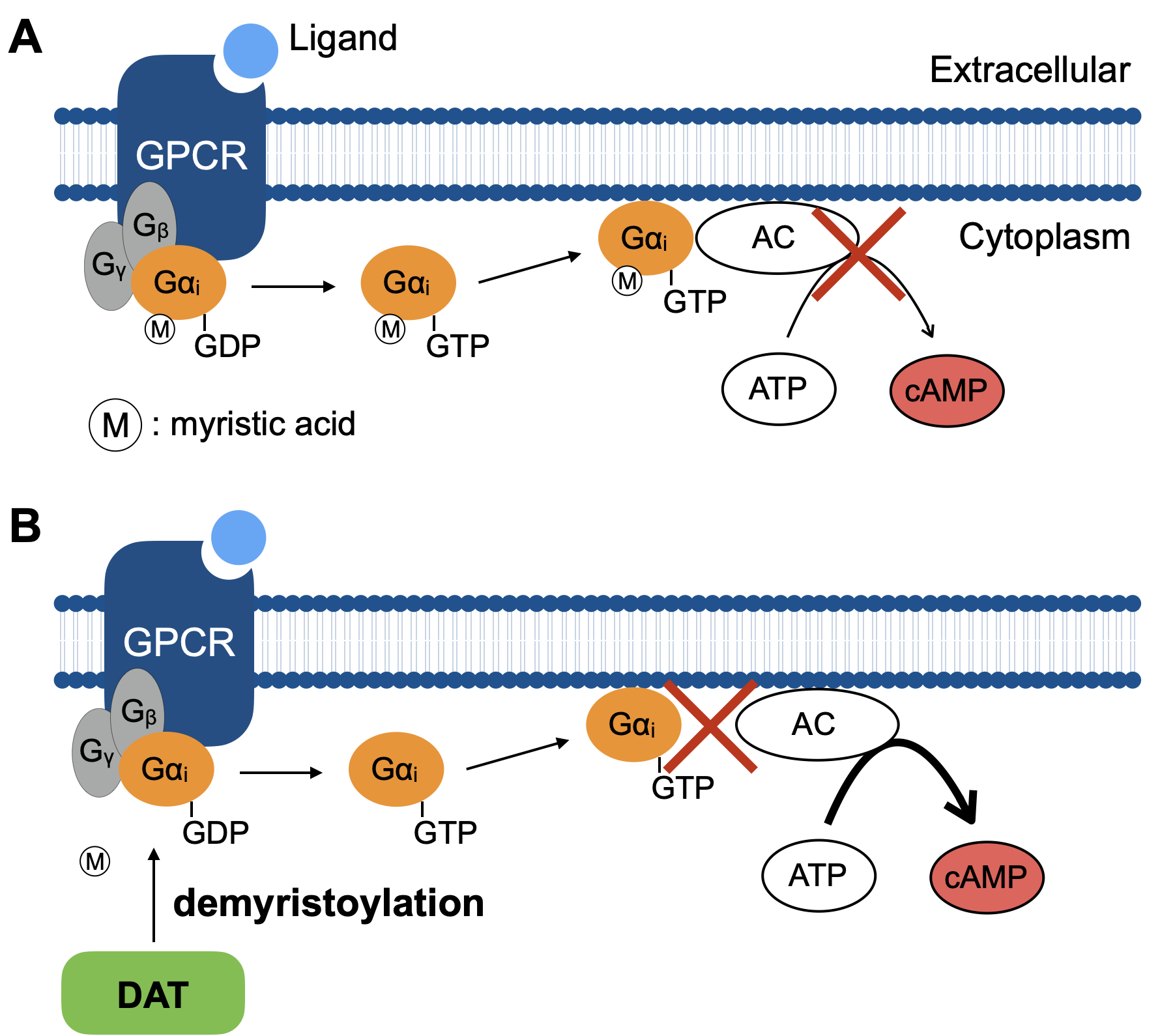
Fig. The mechanism by which DAT of B. parapertussis inactivates heterotrimeric Gi GTPases. (A) Gαi inhibits the activity of adenylate cyclase (AC) and a downstream inhibitory signal in response to extracellular ligand for Gi-coupled GPCR. (B) DAT inactivates heterotrimeric Gi GTPases by inhibiting the interaction of Gαi with AC through the demyristoylation of Gαi.
- Home
- Achievement
- Research Activities
- A novel toxin, inactivating heterotrimeric Gi GTPases, from Bordetella parapertussis (Horiguchi Lab, in PNAS)