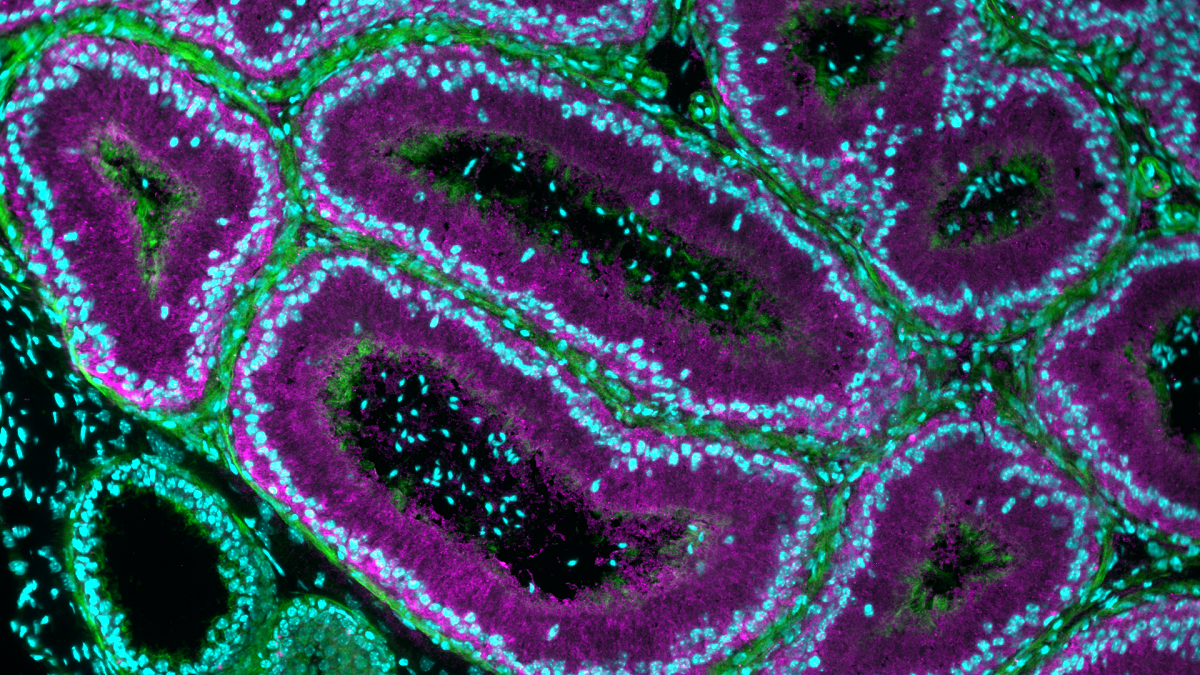
A small secreted protein NICOL regulates lumicrine-mediated sperm maturation and male fertility (Ikawa Lab, in Nat. Commun)
The mammalian spermatozoa produced in the testis require functional maturation in the epididymis for their full competence. Epididymal sperm maturation is regulated by lumicrine signalling pathways in which testis-derived secreted signals relocate to the epididymis lumen and promote functional differentiation. However, the detailed mechanisms of lumicrine regulation are unclear. Herein, we demonstrate that a small secreted protein, NELL2-interacting cofactor for lumicrine signalling (NICOL), plays a crucial role in lumicrine signalling in mice. NICOL is expressed in male reproductive organs, including the testis, and forms a complex with the testis-secreted protein NELL2, which is transported transluminally from the testis to the epididymis. Males lacking Nicol are sterile due to impaired NELL2-mediated lumicrine signalling, leading to defective epididymal differentiation and deficient sperm maturation but can be restored by NICOL expression in testicular germ cells. Our results demonstrate how lumicrine signalling regulates epididymal function for successful sperm maturation and male fertility.
This article was published in Nature Communications, on April 24.
Title: “A small secreted protein NICOL regulates lumicrine-mediated sperm maturation and male fertility”
Authors: Daiji Kiyozumi, Kentaro Shimada, Michael Chalick, Chihiro Emori, Mayo Kodani, Seiya Oura, Taichi Noda, Tsutomu Endo, Martin M. Matzuk, Daniel H. Wreschner, and Masahito Ikawa
DOI: https://doi.org/10.1038/s41467-023-37984-x
Links
- Home
- Achievement
- Research Activities
- A small secreted protein NICOL regulates lumicrine-mediated sperm maturation and male fertility (Ikawa Lab, in Nat. Commun)