Age-related B-cell senescence causes dysbiosis of gut microbiota. (Hara Lab, in Nat. Cell. Biol.)
【Summary】
A research group led by Professor Eiji Hara (concurrently with Immunology Frontier Research Center (IFReC) and Center for Infectious Diseases Education and Research (CiDER), Osaka University), Assistant Professor Shimpei Kawamoto, and Specially Appointed Researcher Ken Uemura of the Department of Molecular Microbiology, Research Institute for Microbial Diseases (RIMD), Osaka University, has revealed that long-term stimulation by gut microbiota causes the induction of cellular senescence in immunoglobulin A (IgA)-producing B cells, resulting in changes in the production and diversity of IgA, which in turn causes dysbiosis of gut microbiota. This research clarifies the mechanism of changes in the gut microbiota with aging. Furthermore, it could lead to the development of methods to improve age-related dysbiosis of the gut microbiota, which is thought to be one of the causes of various age-related diseases.
【Research background】
We all experience aging, a phenomenon in which our biological functions deteriorate. We all want to avoid aging because it leads to a decline in quality of life and the development of severe age-related diseases such as cancer and dementia. Therefore, research has been conducted in many countries with aging populations, including Japan, to extend a healthy life span by slowing the aging process. Recently, it has become clear that one of the causes of aging is the increase of senescent cells, which accumulate in the body with age and secrete various inflammatory substances, thereby accelerating the aging process. However, it has yet to be understood why senescent cells accumulate in the body with aging. On the other hand, the gut microbiota in our intestinal tract, composed of an enormous number and variety of gut bacteria, plays an essential role in maintaining our health. It has become clear that unfavorable changes in the composition of the gut microbiota -dysbiosis- with aging are closely related to the development of various age-related diseases. However, little is known about why the dysbiosis of gut microbiota occurs with aging.
【Research result】
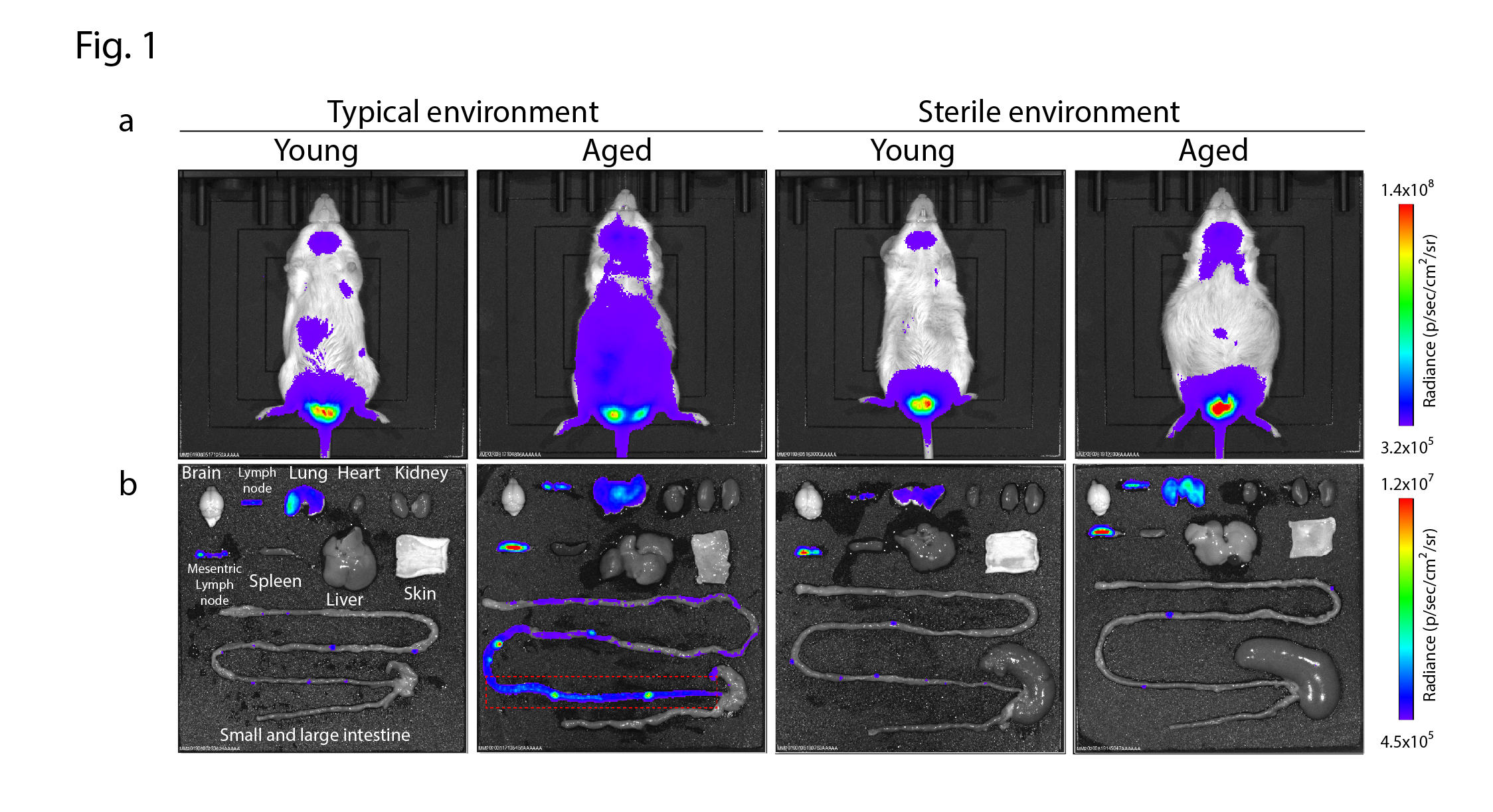
The research group led by Professor Hara, Assistant Professor Kawamoto, and Specially Appointed Researcher Uemura used genetically engineered mice in which senescent cells could be visualized alive in vivo and kept them in a typical environment with bacteria in the gut or a sterile environment with no bacteria in the body for an extended period to observe changes in the accumulation of senescent cells over time. As a result, we noticed that mice bred in a normal environment showed an increase in senescent cells in the abdomen with aging, while mice bred in a sterile environment showed no increase in senescent cells (Fig. 1a). Furthermore when the organs isolated from these mice were examined, significant differences were observed mainly in the ileal part of the gut (Fig. 1b). We then used single-cell RNA sequencing analysis and other techniques to determine which cells in the ileum are inducing cellular senescence. We found that a part of germinal center B cells, which are responsible for immunoglobulin A (IgA) production in the gut, causes cellular senescence. IgA regulates the composition of the gut microbiota by binding to gut bacteria. Therefore, we hypothesized that cellular senescence of germinal center B cells in the ileum with aging might lead to decreased IgA production and abnormal intestinal microflora. We thus followed the same individual mice for two years and analyzed in detail the age-related changes in IgA production and gut microbiota. The results showed that the production of IgA decreases with age, and the binding of IgA to gut bacteria changes, leading to a dysbiosis of gut microbiota. Furthermore, the age-related decline in IgA production and diversity observed in wild-type mice was suppressed in mice where cellular senescence was prevented. In addition, it was found that B cells isolated from wild-type mice show a decrease in IgA production capacity and a marked reduction in the ability to control the gut microbiota as they age, while B cells isolated from mice with reduced susceptibility to cellular senescence show no decrease in IgA production capacity and maintain some ability to control the gut microbiota even as they age.
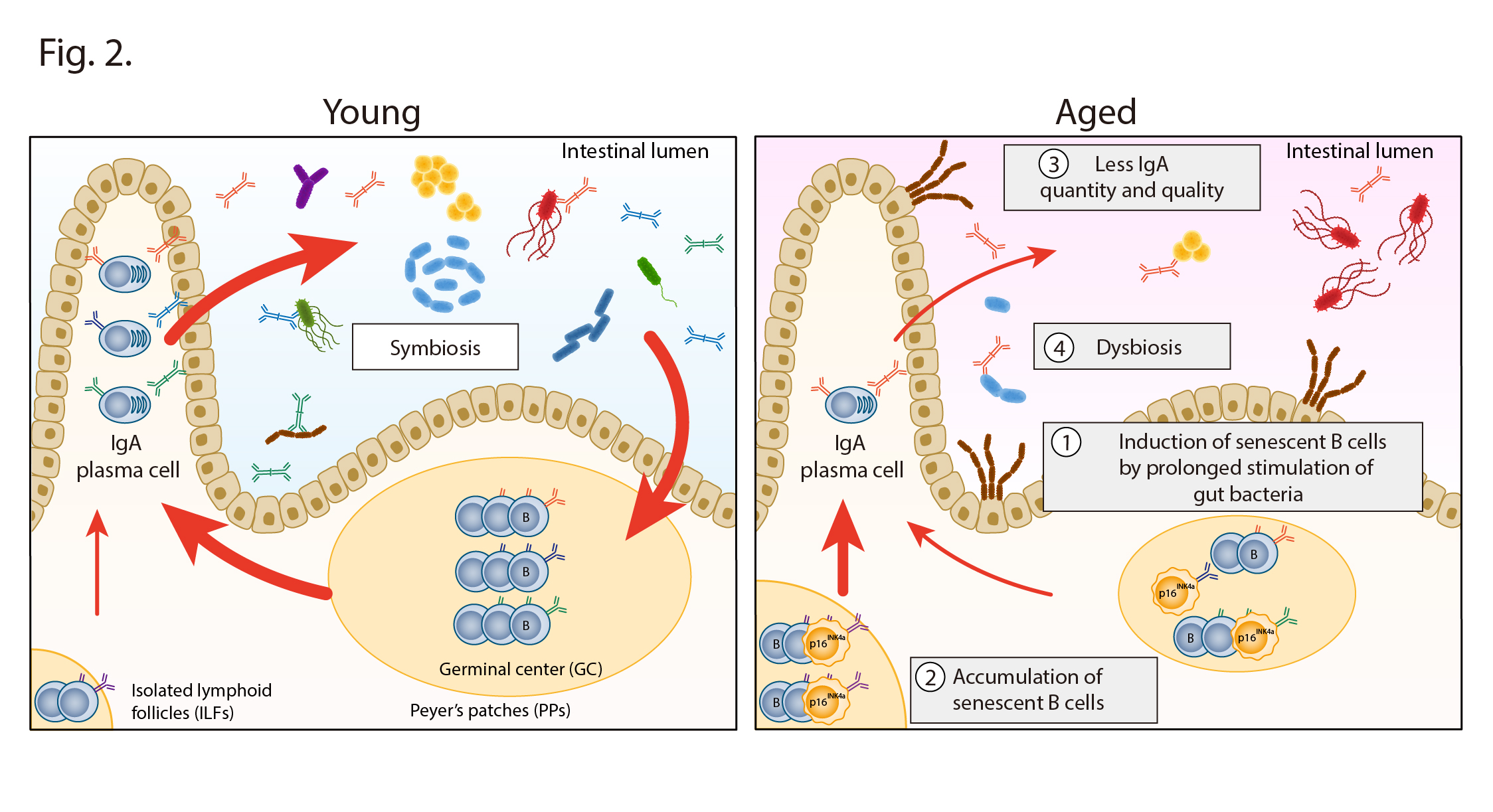
In conclusion, it is clear that in young mice, gut homeostasis is maintained by controlling the composition of the gut microbiota through IgA (Fig. 2 left), but when stimulated by gut bacteria over a long period during aging, the germinal center B cells in the ileum cause cellular senescence, resulting in a decrease in IgA quantity and diversity, which in turn provokes dysbiosis in the gut microbiota (Fig. 2 right).
【Future perspective】
This study revealed that the gut microbiota, which is supposed to have a symbiotic relationship with the host, becomes stressful for the host in the long term, inducing cellular senescence of germinal center B cells in the ileum, which in turn causes dysbiosis of the gut microbiota. This discovery is expected to elucidate the causes of age-related abnormalities in gut microbiota, which have long been unknown, and to develop new methods to prevent abnormalities in gut microbiota, thereby contributing to the extension of healthy life expectancy. In the future, we would like to investigate whether the same phenomenon occurs in humans and to develop a safe and effective method to inhibit the induction of cellular senescence in germinal center B cells.
This research was published online in the journal Nature Cell Biology on 12, 2023.
Title: Bacterial induction of B-cell senescence promotes age-related changes in the gut microbiota
Authors: Shimpei Kawamoto, Ken Uemura, Nozomi Hori, Lena Takayasu, Yusuke Konishi, Kazutaka Katoh, Tomonori Matsumoto, Masae Suzuki, Yusuke Sakai, Tatsuyuki Matsudaira, Takahiro Adachi, Naoko Ohtani, Daron M. Standley, Wataru Suda, Shinji Fukuda & Eiji Hara
Links
- Home
- Achievement
- Research Activities
- Age-related B-cell senescence causes dysbiosis of gut microbiota. (Hara Lab, in Nat. Cell. Biol.)