1700029I15Rik orchestrates the biosynthesis of acrosomal membrane proteins required for sperm–egg interaction (Ikawa Lab, in PNAS)
A study led by Assistant Professor Yonggang Lu and Professor Masahito Ikawa in the Department of Experimental Genome Research has revealed that a testis-specific type II transmembrane protein, 1700029I15Rik, is essential for the biosynthesis of acrosomal membrane glycoproteins required for sperm–egg fusion.
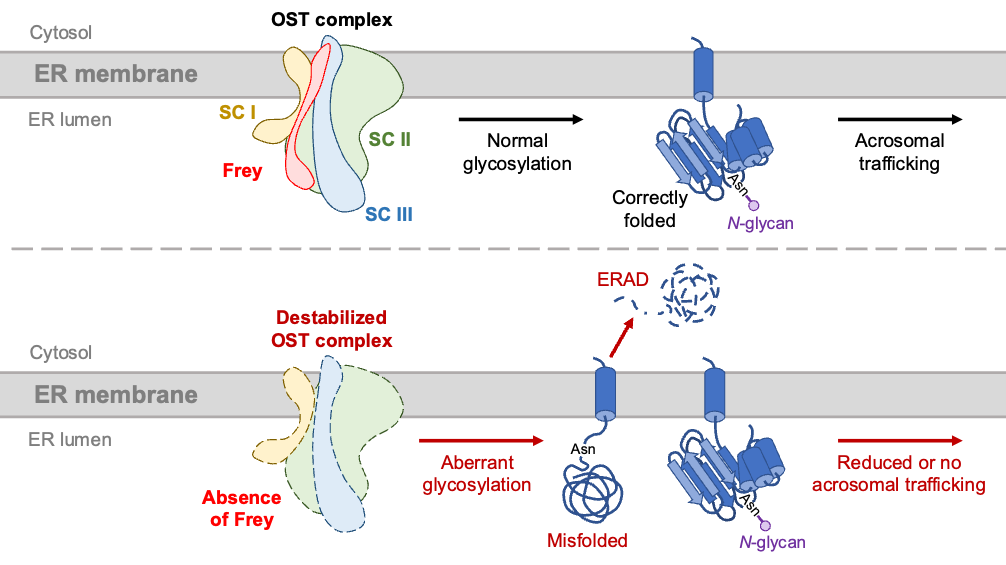
Researchers in the Ikawa Lab pioneer the study of mammalian fertilization by the discovery of multiple gamete fusion-required sperm proteins, including IZUMO1 (Nature 2005), FIMP (PNAS 2020a), SOF1, TMEM95, SPACA6 (PNAS 2020b), and DCST1/2 (Commun Biol 2022). Among these proteins, IZUMO1, TMEM95, SPACA6, and DCST1/2 are glycoproteins localized to the sperm acrosomal membrane. About three decades ago, Professor Ikawa reported that the testis-specific endoplasmic reticulum (ER) chaperone Calmegin mediates the processing of sperm plasma membrane (Nature 1997). However, depletion of Calmegin does not affect sperm–egg fusion, suggesting that the fusion-related sperm proteins are processed via an unknown, independent pathway.
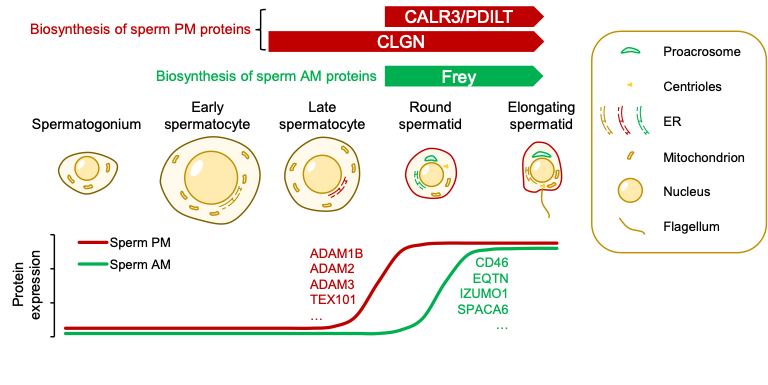
In this study, Yonggang Lu and colleagues discovered that 1700029I15Rik, an ER protein predominantly expressed in round spermatids, specifically governs the processing of acrosomal membrane glycoproteins by interacting with and stabilizing the oligosaccharyltransferase (OST) complex subunits pivotal for N-glycosylation. 1700029I15Rik knockout male mice exhibit normal expression of sperm plasma membrane proteins but reduced abundance of sperm acrosomal membrane glycoproteins. SPACA6 becomes absent in 1700029I15Rik knockout sperm, providing a direct explanation for the defective sperm fertilizing ability. Due to aberrant accumulation of misfolded proteins, the ubiquitin-dependent ER-associated degradation (ERAD) machinery, as well as ubiquitinated proteins, are upregulated in 1700029I15Rik knockout sperm.
The researchers propose that during spermiogenesis, the biosynthesis of proteins destined for different subcellular compartments is orchestrated in a spatiotemporal manner. Given that 1700029I15Rik is highly conserved in humans, this discovery in mice may provide profound insights into the etiology of idiopathic male infertility and the development of a non-hormonal contraceptive approach involving molecular interventions in the biosynthesis of acrosomal membrane proteins.
This study was published in PNAS, on Feb 14, 2023.
Title: 1700029I15Rik orchestrates the biosynthesis of acrosomal membrane proteins required for sperm–egg interaction
Authors: Yonggang Lu, Kentaro Shimada, Shaogeng Tang, Jingjing Zhang, Yo Ogawa, Taichi Noda, Hiroki Shibuya, Masahito Ikawa
Links
- Home
- Achievement
- Research Activities
- 1700029I15Rik orchestrates the biosynthesis of acrosomal membrane proteins required for sperm–egg interaction (Ikawa Lab, in PNAS)