Human sperm TMEM95 binds eggs and facilitates membrane fusion (Ikawa Lab, in PNAS)
A research led by Assistant Professor Yonggang Lu and Professor Masahito Ikawa in the Department of Experimental Genome Research, in collaboration with Postdoctoral Fellow Shaogeng (Steven) Tang and Professor Peter S. Kim at Stanford University, has revealed that human sperm TMEM95 binds the egg plasma membrane and facilitates membrane fusion.
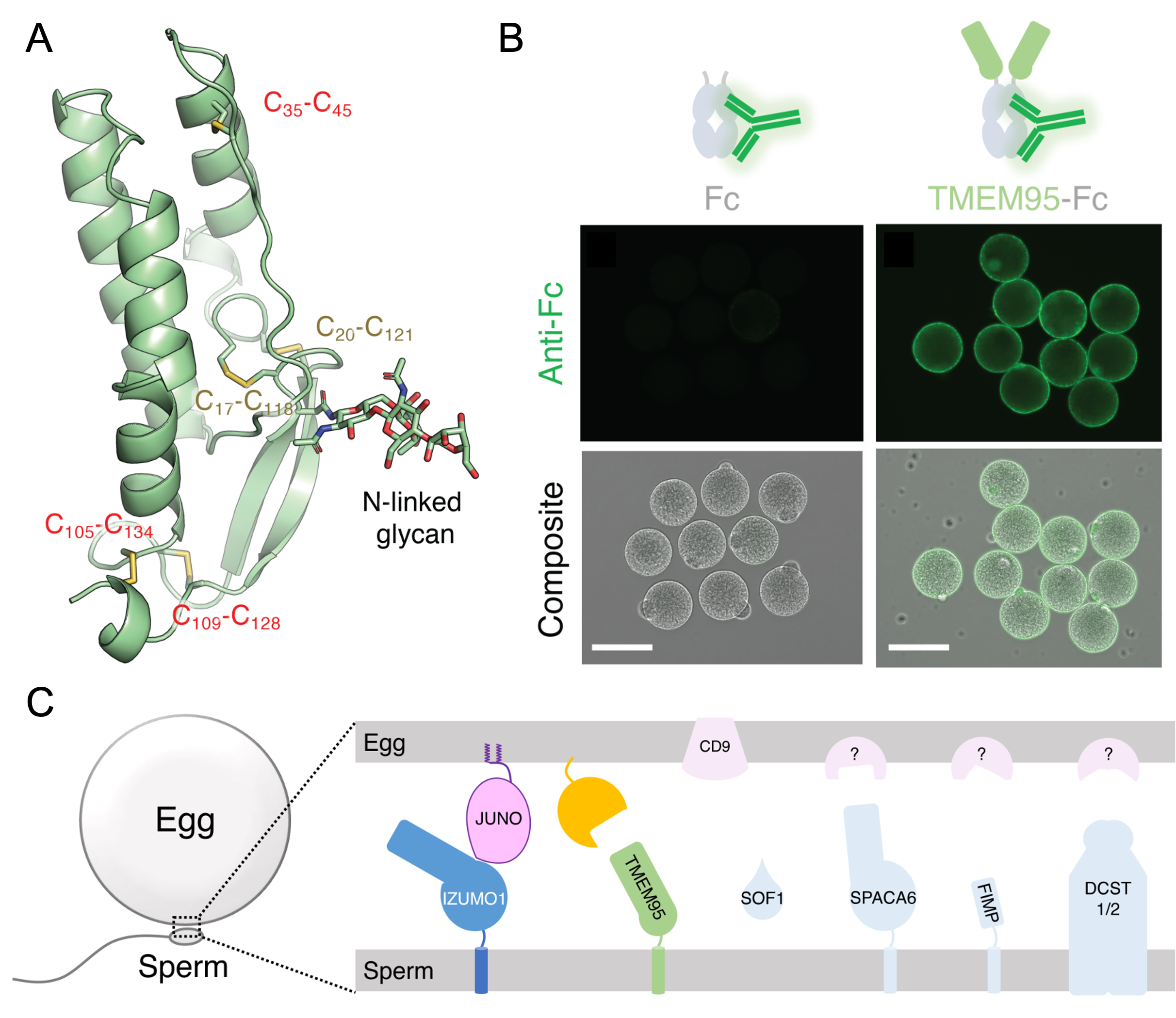
In 2005, the laboratory discovered IZUMO1, the first sperm membrane protein essential for sperm fusion with eggs. In recent years, using the CRISPR/Cas9-mediated genome editing in mice, they further identified FIMP, SOF1, TMEM95, SPACA6, and DCST1/2 as indispensable sperm factors for fertilization and sperm-egg interaction. On the other hand, JUNO has been identified as IZUMO1’s receptor on the egg surface, but the existence of receptors for the other sperm factors has remained unknown. In this study, by X-ray crystallography, the team revealed that the ectodomain of human TMEM95, similar to the N-termini of IZUMO1 and SPACA6, is composed of an α-helix bundle and a β-hairpin. Next, by exploiting the nature that human sperm can fuse with the eggs of golden hamsters, the molecular function of human TMEM95 was interrogated in vitro. As a result, they discovered that TMEM95 has a counterpart on the egg plasma membrane. Furthermore, monoclonal antibodies against TMEM95 inhibited the fusion between human sperm and hamster eggs without interfering with their binding, suggesting that the interaction between TMEM95 and its egg receptor may have a direct role in membrane fusion. The authors anticipate that this work would have profound implications for the diagnosis of idiopathic male infertility and the rational development of clinical treatment.
This Article was published in PNAS in Oct 4, 2022.
Title: Human sperm TMEM95 binds eggs and facilitates membrane fusion
Authors: Shaogeng Tang*, Yonggang Lu*, Will M Skinner, Mrinmoy Sanyal, Polina V Lishko, Masahito Ikawa#, Peter S Kim# (*: 1st Author, #: Corresponding Author)
Links
- Home
- Achievement
- Research Activities
- Human sperm TMEM95 binds eggs and facilitates membrane fusion (Ikawa Lab, in PNAS)