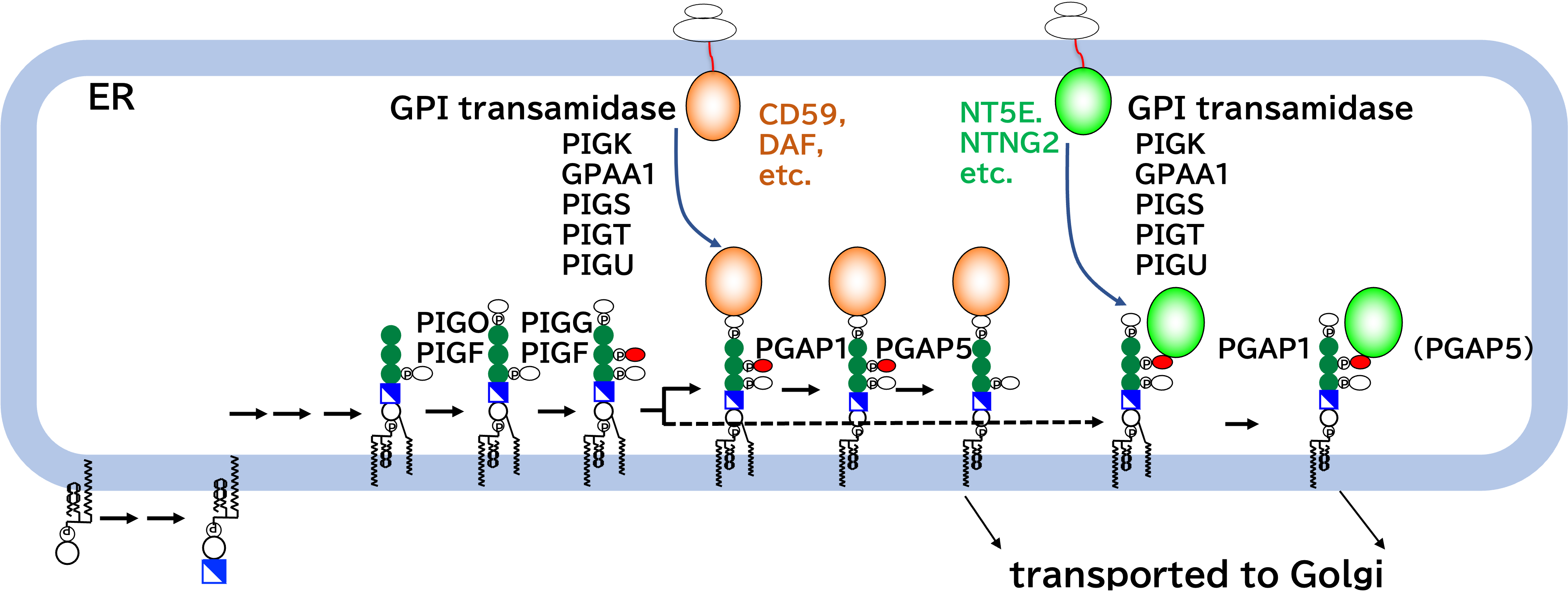
Ethanolamine-phosphate on the second mannose is a preferential bridge for some GPI-anchored proteins. (Kinoshita Lab, EMBO Rep.)
Glycosylphosphatidylinositols (GPIs) are glycolipids that anchor many proteins (GPI-APs) on the cell surface. The core glycan of GPI precursor has three mannoses, which in mammals, are all modified by ethanolamine-phosphate (EthN-P). It is postulated that EthN-P on the third mannose (EthN-P-Man3) is the bridge between GPI to the protein and the second (EthN-P-Man2) is removed after GPI-protein attachment. However, EthN-P-Man2 may not be always transient, as mutations of PIGG, the enzyme that transfers EthN-P to Man2, result in inherited GPI deficiencies (IGDs), characterized by neuronal dysfunctions. Here, we show EthN-P on Man2 is the preferential bridge in some GPI-APs, among them, the ect-5’-nucleotidase and netrin G2. We found that CD59, a GPI-AP, is attached via EthN-P-Man2 both in PIGB-knockout cells, in which GPI lacks Man3 and with a small fraction, in wild-type cells. Our findings modify the current view of GPI anchoring and provide mechanistic bases of IGDs caused by PIGG mutations.
This article is published online in EMBO Reports on May, 2022
Title: Ethanolamine-phosphate on the second mannose is a preferential bridge for some GPI-anchored proteins.
Authors: Mizuki Ishida, Yuta Maki, Akinori Ninomiya, Yoko Takada, Philippe Campeau, Taroh Kinoshita, Yoshiko Murakami*
EMBO Rep. 2022 May 23:e54352. doi:10.15252/embr.202154352.
Links
- Home
- Achievement
- Research Activities
- Ethanolamine-phosphate on the second mannose is a preferential bridge for some GPI-anchored proteins. (Kinoshita Lab, EMBO Rep.)