The role of Gal-3 in maintaining the quiescent state of hematopoietic stem cells (Takakura Lab, in Nat Commun)
The research group in the Department of Signal Transduction, researcher Weizhen Jia and professor Nobuyuki Takakura clarified the role of galactose-binding lectin-3 (Gal-3) in maintaining the quiescent state of hematopoietic stem cells (HSCs) and its molecular mechanism.
HSCs are the source of blood supply. Excessive division of HSCs will cause cell exhaustion. Therefore, it is very important to maintain the balance between self-replication and differentiation of HSCs. However, the cellular and molecular mechanism coordinating the balance between HSC quiescence and differentiation is not fully understood.
As a consequence of analyzing the molecular mechanism for HSC quiescence, the research group found that Gal-3 is highly expressed in quiescent HSCs. Gal-3 is a member of galactose binding lectin. It participates in various biological phenomenon such as development, differentiation, cell cycle, and apoptosis, etc. Their research found that the percentage of long-term HSCs (LT-HSC) in the G0 phase decreased in BM from Gal-3 KO relative to wild-type mice and that reconstruction ability of LT-HSCs in Gal-3 KO mice was attenuated. These results illustrate that Gal-3 regulates the cell-cycle of HSCs and plays a critical role in maintaining the quiescent state.
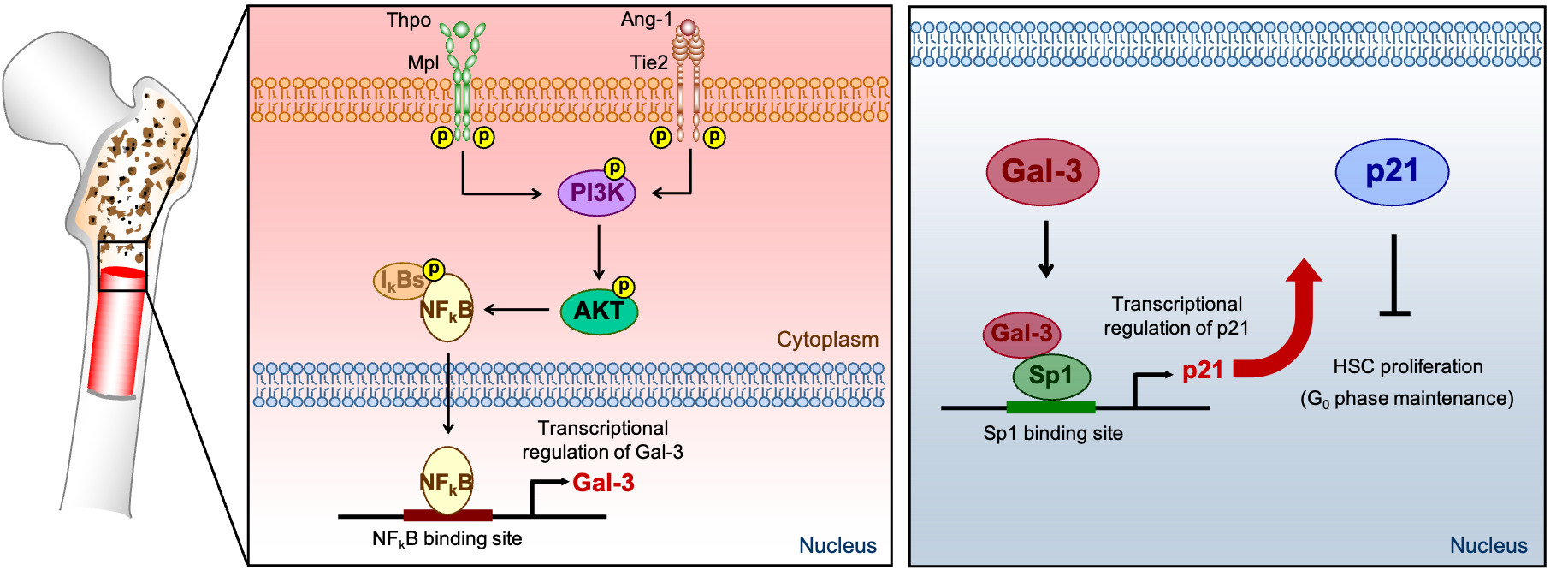
In terms of the mechanism of how Gal-3 maintains HSC quiescence, the researchers found that Angiopoietin-1 or Thrombopoietin, secreted from bone marrow niche cells, binds to the receptor Tie2 or Mpl on the surface of stem cells. Nuclear translocation of NF-κB mediated by PI3K/AKT pathway following to stimulation by Tie2 or Mpl expressed in HSCs promotes the production of Gal-3, which then binds to Sp1 and induces p21 transcription resulting in the inhibition of cell cycle progression and maintenance of LT-HSC quiescence.
This research clarified a novel mechanism of bone marrow niche for the maintenance of HSCs and may contribute to stem cell expansion in culture and bone marrow transplantation, as well as promoting leukemia cancer stem cell-targeted therapy and other clinical applications.
Links
-
Figure: Proposed model showing the molecular mechanisms essential for maintaining LT-HSC quiescence. Angiopoietin-1 (Ang-1) or Thrombopoietin (Thpo) secreted from bone marrow niche cells, binds to the receptor Tie2 or Mpl on the surface of LT-HSCs. Nuclear translocation of NF-κB mediated by activated AKT following stimulation by Tie2 or Mpl expressed in LT-HSCs promotes the production of Gal-3, which then binds to Sp1 and induces p21 transcription, resulting in the inhibition of cell cycle progression and maintenance of LT-HSC quiescence.
- Home
- Achievement
- Research Activities
- The role of Gal-3 in maintaining the quiescent state of hematopoietic stem cells (Takakura Lab, in Nat Commun)