Takakura Lab/Division of Cellular and Molecular Biology Department of Signal Transduction
Tissue-specific stem cells continuously produce terminally differentiated functional cells and maintain organ integrity. Blood vessels supply oxygen and nutrients to all tissues; tissues and organs cannot develop without blood vessel formation. Our aim is to elucidate the cellular and molecular mechanisms underlying vascular formation (particularly those involving stem cells) and to develop strategies to manage patients with vascular diseases.
Mechanism of vascular formation
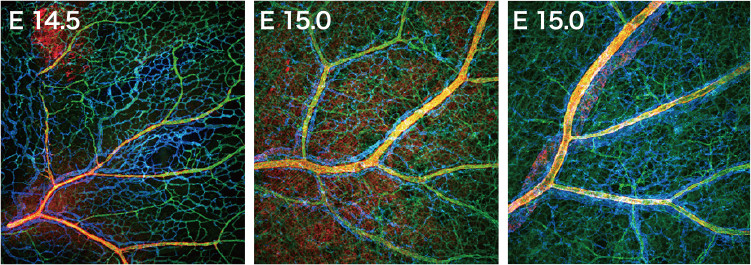
Tissue homeostasis in all organs is maintained via a highly hierarchal architecture of blood vessels, which is precisely regulated in an organ-specific manner. We are examining how blood vessel diversity is regulated, focusing on the processes of angiogenesis and blood vessel maturation. Our recent studies clarified that arterial-venous alignment is regulated by the apelin/APJ system and is critical for thermoregulation (Kidoya, Dev Cell 2015).
Stemness and vascular niche
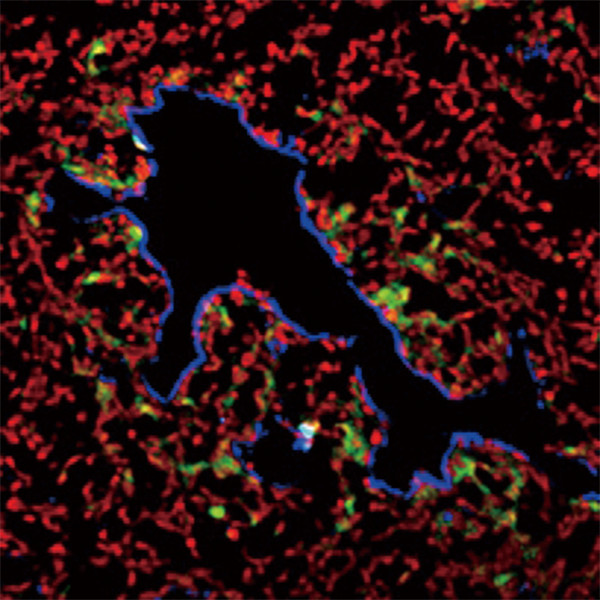
Stem cells localize in perivascular areas in many organs. Cells that comprise such a vascular niche regulate the “stemness” of stem cells. In our cancer stem cell (CSC) model based on PSF1 promoter activity, we found that CSCs proliferate and survive in the vascular niche (Nagahama, Cancer Res 2010, Kinugasa, Stem Cells 2014). Regulation of the vascular niche is a promising approach to inhibiting tumor growth. Because blood vessels developing in the tumor microenvironment are immature and abnormal, normalization of blood vessel development must control CSCs in the vascular niche. Vascular normalization also improves anti-tumor immunity and drug delivery. Therefore, we are seeking ways to normalize blood vessels within tumors.
Development of tissue regeneration methods based on endothelial stem cells
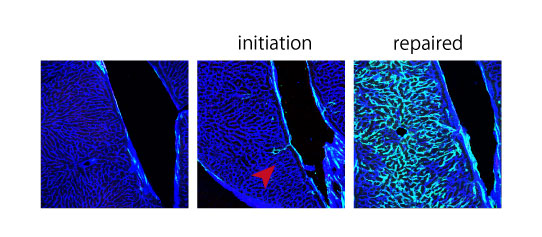
We have identified endothelial stem cells in pre-existing blood vessels and showed their utility for vascular regeneration (Naito, EMBO J 2012). Recently, we found that such endothelial stem cells affect the resistance of cancer cells to anti-angiogenic therapy (Naito, Cancer Res 2016). We are examining how endothelial stem cells develop and how they are maintained during development with a view to using this cell population to treat vascular disease.
-
Fig. 1.Vascular development in mouse embryos. Hierarchal architecture of blood vessels accompanied by arterial (yellow)-venous (blue) alignment. Green; endothelial cells.
-
Fig. 2.Endothelial cells (blue) and CSCs (green) in a tumor. CSCs localize at the perivascular area, the so called "vascular niche."
-
Fig. 3. Linage tracing of VESC. New blood vessels emerged from VESC (shown in green) Most of the endothelial cells (ECs) are replaced by ECs drived from VESC.
Staff
- Prof. : Nobuyuki Takakura
- Asst. Prof. : Fumitaka Muramatsu
- Asst. Prof.:Jia Wei Zhen
- Asst. Prof. :Kensuke Hachiya
- SA Asst. Prof. : Bal Zeynep (concur.)
- SA Asst. Prof. : Fitriana Nur Rahmawati
- JSPS Postdoc.: Yoshimi Noda
Website
Publications
(1) Indispensable role of Galectin-3 in promoting quiescence of hematopoietic stem cells. Jia W. et al., Nature Commun. (2021) 12(1):2118.
(2)Regnase-1-mediated post-transcriptional regulation is essential for hematopoietic stem and progenitor cell homeostasis. Kidoya H. et al., Nat Commun. (2019) 10(1):1072.
(3) TAK1 prevents endothelial apoptosis and maintains vascular integrity. Naito H., et al., Dev Cell. (2019) 48(2):151-166.e7.
(4) CD157 marks tissue-resident endothelial stem cells with homeostatic and regenerative properties. Wakabayashi T., et al., Cell Stem Cell 22(3):384-397, 2018.
(5) APJ regulates parallel juxtapositional alignment of arteries and veins in the skin. Kidoya H., et al., Dev Cell (2015) 33(3):247-59.
(6) A role for hematopoietic stem cells in promoting angiogenesis. Takakura N., et al., Cell (2000) 102(2):199-209.
- Home
- Laboratories
- Takakura Lab