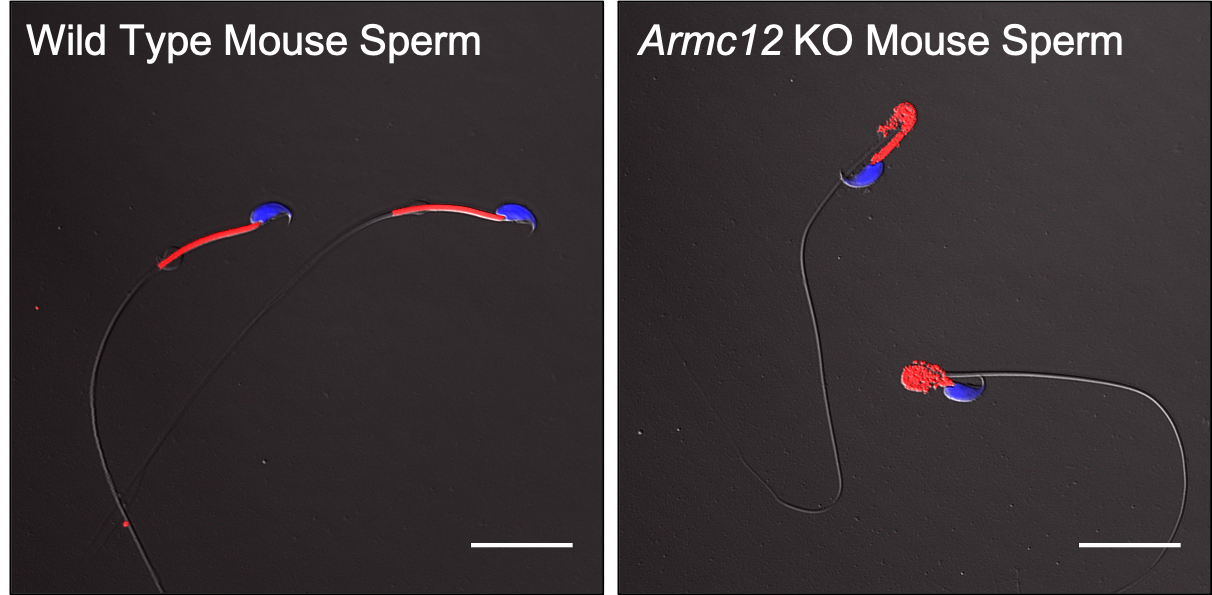
ARMC12 regulates spatiotemporal mitochondrial dynamics during spermiogenesis and is required for male fertility (Ikawa Lab, PNAS)
The mammalian sperm midpiece has a unique double helical structure called the mitochondrial sheath that wraps tightly around the axoneme. Despite the remarkable organization of the mitochondrial sheath, the molecular mechanisms involved in mitochondrial sheath formation are unclear. In the process of functional screens of testis-enriched genes in mice, we identified armadillo repeat containing 12 (ARMC12) as an essential protein for mitochondrial sheath formation. Here, we engineered Armc12 null mice, FLAG-tagged Armc12 knock-in mice and TBC1 domain family member 21 (Tbc1d21) null mice to define the functions of ARMC12 in mitochondrial sheath formation in vivo. We discovered that absence of ARMC12 causes abnormal mitochondrial coiling along the flagellum (Fig.1), resulting in reduced sperm motility and male sterility.
During spermiogenesis, sperm mitochondria in Armc12 null mice cannot elongate properly at the mitochondrial interlocking step which disrupts abnormal mitochondrial coiling (Fig.2).
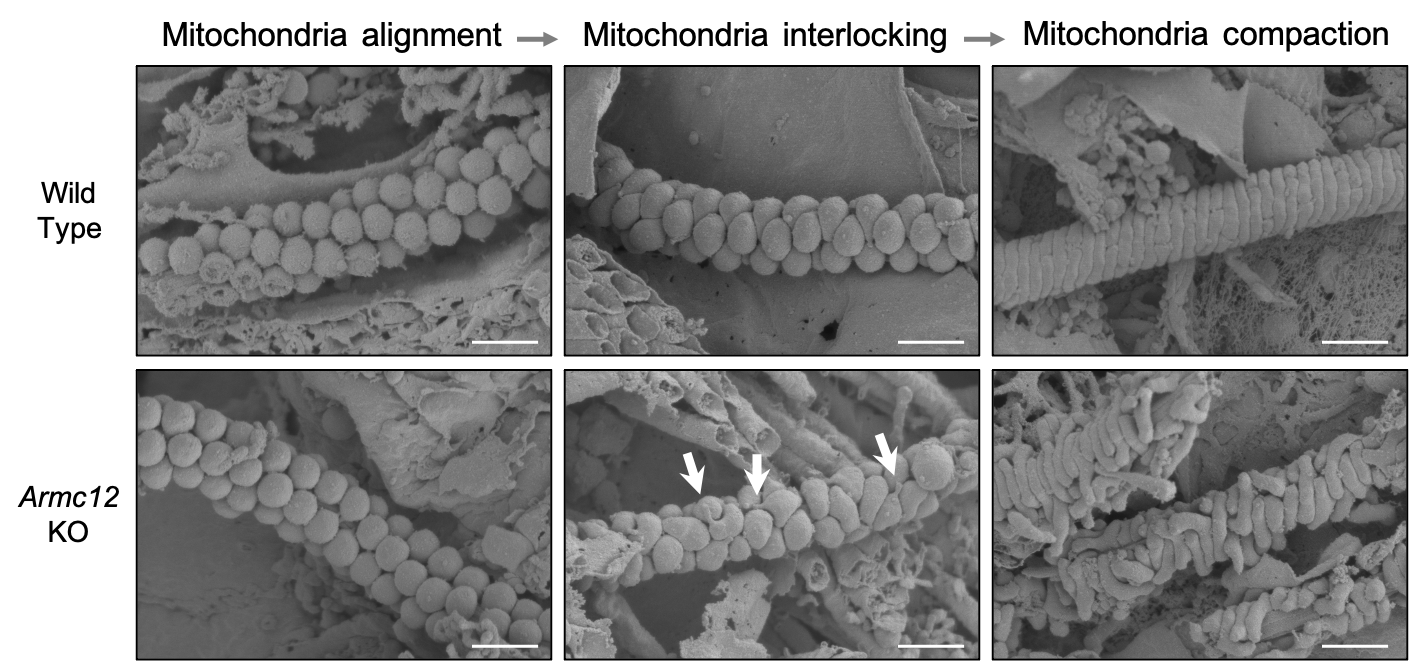
Fig.2 Development of mitochondrial sheath during spermiogenesis observed by SEM.
ARMC12 is a mitochondrial peripheral membrane protein and functions as an adherence factor between mitochondria in cultured cells. ARMC12 in testicular germ cells interacts with mitochondrial proteins MIC60, VDAC2 and VDAC3 as well as TBC1D21 and GK2, which are required for mitochondrial sheath formation. We also observed that TBC1D21 is essential for the interaction between ARMC12 and VDAC proteins in vivo. These results indicate that ARMC12 uses integral mitochondrial membrane proteins VDAC2 and VDAC3 as scaffolds to link mitochondria and works cooperatively with TBC1D21. Thus, our studies have revealed that ARMC12 regulates spatiotemporal mitochondrial dynamics to form mitochondrial sheath through cooperative interactions with several proteins on the sperm mitochondrial surface (Fig.3).
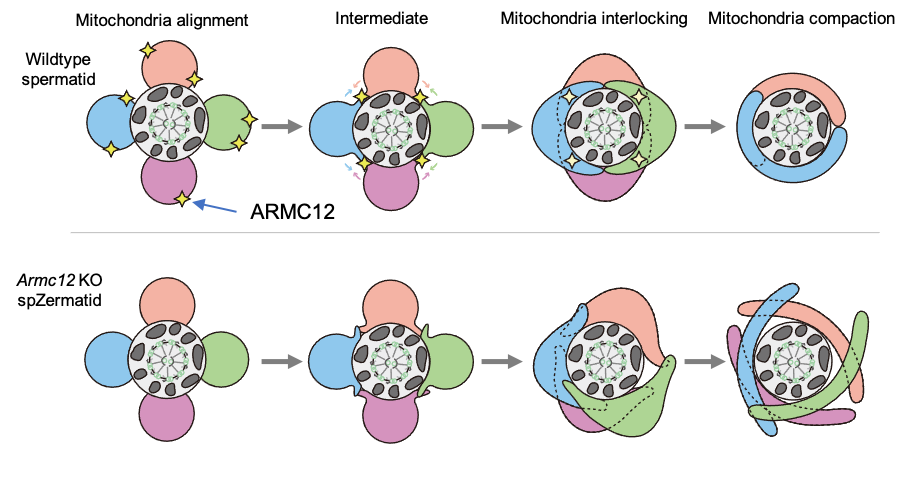
Fig.3 Schematic models for the function of ARMC12.
Significance Statement
Although formation of the mitochondrial sheath is a critical process in the formation of mature spermatozoa, the molecular mechanisms involved in mitochondrial sheath genesis remain unclear. Using gene-manipulated mice, we discovered that ARMC12 regulates spatiotemporal “sperm mitochondrial dynamics” during mitochondrial sheath formation through interactions with mitochondrial proteins MIC60, VDAC2 and VDAC3 as well as testis-specific proteins TBC1D21 and GK2. In addition, we demonstrated that ARMC12-interacting proteins TBC1D21 and GK2 are also essential for mitochondrial sheath formation. Our manuscript sheds new light on the molecular mechanisms of mitochondrial sheath formation and the regulation of sperm mitochondrial dynamics, allowing us to further understand the biology of spermatogenesis and the etiology of infertility in men.
This article was published in PNAS, on Feb 2nd, 2021.
Title: “ARMC12 regulates spatiotemporal mitochondrial dynamics during spermiogenesis and is required for male fertility”
Authors: Keisuke Shimada, Soojin Park, Haruhiko Miyata, Zhifeng Yu, Akane Morohoshi, Seiya Oura, Martin M. Matzuk and Masahito Ikawa.
Links
- Home
- Achievement
- Research Activities
- ARMC12 regulates spatiotemporal mitochondrial dynamics during spermiogenesis and is required for male fertility (Ikawa Lab, PNAS)