Novel switch protein that ‘turns on’ sperm for fertilization (Ikawa-Lab, in Science)
Researchers from Osaka University and Baylor College of Medicine identify a protein secreted by the testis that travels downstream through the lumen to differentiate the epididymis and mature sperm.
Osaka, Japan and Houston, Texas, USA – For a sperm produced in the testis of a man to fertilize a woman’s egg, the sperm must first mature in a man’s epididymis. Now, an international team of researchers has identified a chain of events in which a protein secreted by the testis travels in the luminal fluid, binds to a receptor on the epididymis to induce its differentiation and secretion of a second protein that matures the sperm and enables each sperm to be motile in females.
In a new study published in Science, researchers from Osaka University and Baylor College of Medicine have identified NELL2, a secreted protein factor that acts on the epididymis through this novel “lumicrine” pathway to mediate sperm maturation.
Sperm are produced in the seminiferous tubules of the testis and transit through the epididymis, a long, convoluted tube linked to the vas deferens. When the sperm enter the epididymis, they are not motile and are incapable of fertilization; however, in their passage through the epididymis, the sperm are provided an appropriate environment for maturation and storage pending ejaculation.
It has been hypothesized that proteins released by the testis (upstream) could act on the epididymis (downstream); however, until now, the proteins working through this intriguing lumicrine system of signaling have remained elusive. While it was known that the orphan receptor tyrosine kinase ROS1 expressed in the initial segment of the epididymis is necessary for its differentiation, neither the testicular factors that regulate initial segment differentiation nor the process of sperm maturation had been fully understood.
The researchers zeroed in on NELL2, secreted by testicular germ cells, as a putative lumicrine regulator of fertility. “Using innovative genome editing technology, we generated knockout mice lacking the Nell2 gene and showed that these knockout males are sterile due to a defect in sperm motility,” explains Daiji Kiyozumi, lead author. “Moreover, their infertility could be rescued with a germ-cell-specific transgene, thus excluding other sites of expression. We also illustrated lumicrine signaling by demonstrating tagged NELL2 in the epididymal lumen.”
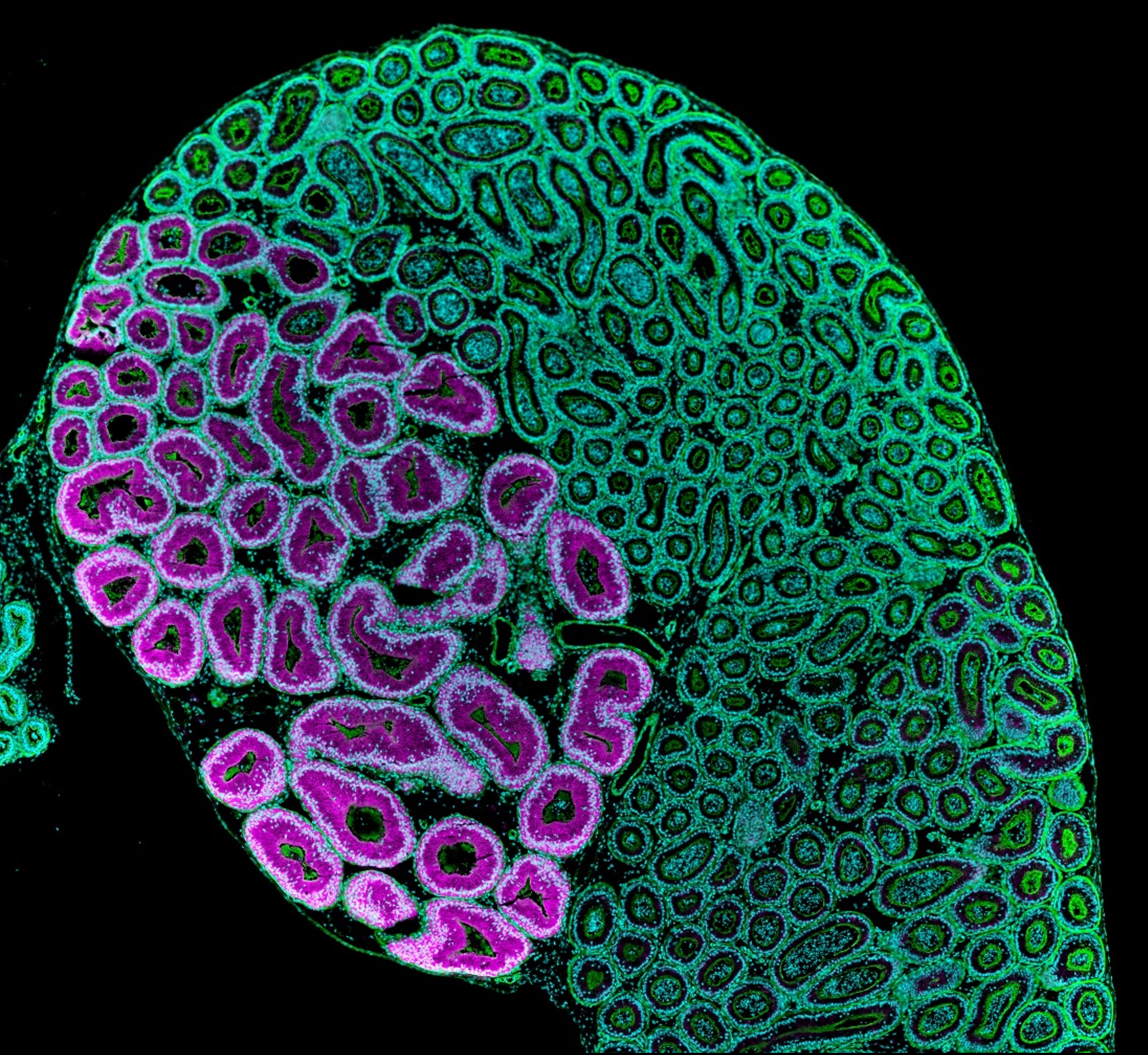
The research team observed that spermatogenesis proceeds normally in Nell2 knockout mouse testes but their epididymis was poorly differentiated, similar to Ros1 knockout mice. Following mating, neither Nell2 knockout nor Ros1 knockout spermatozoa can enter the uterine tubes or fertilize an egg. Further investigation showed that the Nell2 knockout epididymis is incapable of making a key protease, OVCH2, that processes a sperm surface protein, ADAM3, essential for male fertility.
Elaborating on the significance of these novel studies, Professors Masahito Ikawa and Martin M. Matzuk, senior authors, say, “We discovered a complicated cascade of events in which disruption of any point in this lumicrine pathway causes a male to be infertile. Our findings have important translational implications for diagnostic and therapeutic research in male infertility and male contraceptive development. This unique transluminal communication pathway between tissues and organs likely functions elsewhere in our bodies.”
This male infertility and male contraceptive research has been funded by the Japan Society for the Promotion of Science, Ministry of Education, Culture, Sports, Science and Technology, the Japan Agency for Medical Research and Development, the Takeda Science Foundation, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the Bill and Melinda Gates Foundation.
Links
-
Figure1. The proximal segment of the epididymis responds to the switch factor NELL2 and synthesizes OVCH2 (magenta), a protein indispensable for sperm maturation.
-
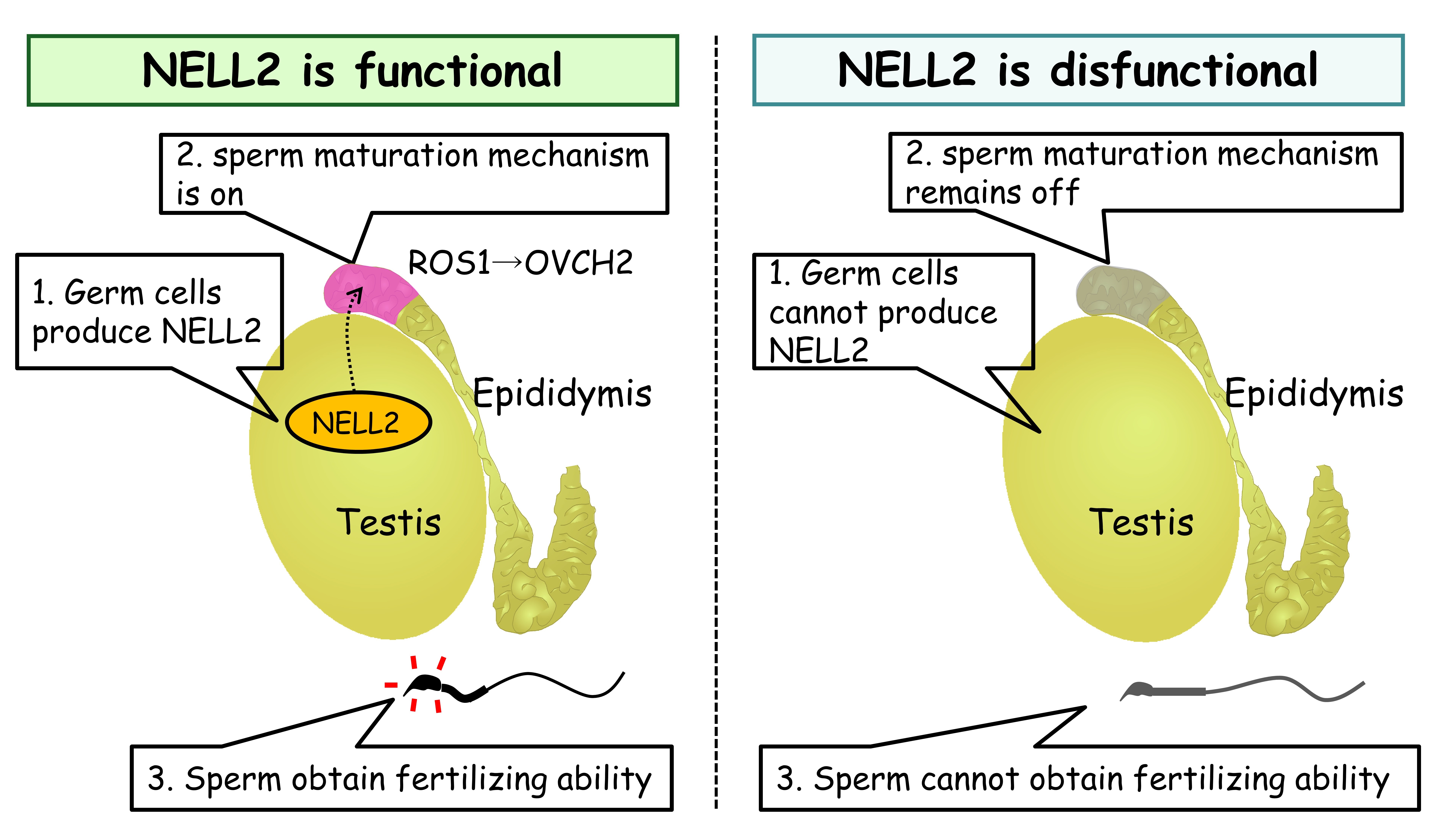
Figure 2. The function of mouse NELL2 Left, 1, Germ cells produce NELL2 during spermatogenesis; 2, In response to NELL2 coming from testis, the sperm maturation machinery turns on in the epididymis; 3, Sperm obtain fertilizing ability during when they migrate through epididymis. Right, In the absence of NELL2, sperm are still generated in the testis but sperm maturation does not occur in the epididymis and males become infertile.
- Home
- Achievement
- Research Activities
- Novel switch protein that ‘turns on’ sperm for fertilization (Ikawa-Lab, in Science)