Discovery of sperm membrane protein FIMP involved in membrane fusion during fertilization (Ikawa Lab, in PNAS)
ABSTRACT
Sperm-oocyte fusion is a critical event in mammalian fertilization, categorized by three indispensable proteins. Sperm membrane protein IZUMO1 and its counterpart oocyte membrane protein JUNO make a protein complex allowing sperm to interact with the oocyte, and subsequent sperm-oocyte fusion. Oocyte tetraspanin protein CD9 also contributes to sperm-oocyte fusion. However, the fusion process cannot be explained solely by these three essential factors. In this study, we focused on analyzing a novel testis-specific gene 4930451I11Rik and generated mutant mice using the CRISPR/Cas9 system. Although IZUMO1 remained in 4930451I11Rik knockout (KO) spermatozoa, the KO spermatozoa were unable to fuse with oocytes and the KO males were severely subfertile. 4930451I11Rik encodes two isoforms, a transmembrane (TM) form and a secreted form. Both CRISPR/Cas9-mediated TM deletion and transgenic (Tg) rescue with the TM form revealed that only the TM form plays a critical role in sperm-oocyte fusion. Thus, we renamed this TM form as Fertilization Influencing Membrane Protein (FIMP). The mCherry-tagged FIMP TM form was localized to the sperm equatorial segment where the sperm-oocyte fusion event occurs. Thus, FIMP is a novel sperm-specific transmembrane protein that is necessary for the sperm-oocyte fusion process.
SIGNIFICANCE STATEMENT
As the human body is comprised of 60 trillion cells that originate from a fertilized egg, sperm-oocyte fusion is the initial event of our life. Few sperm-oocyte fusion factors have been unveiled to date, and only IZUMO1 has been identified as a sperm-specific fusion-mediating protein. Here, we identified the testis-specific 4930451I11Rik gene to be important for male fertility, playing a role in sperm-oocyte fusion during fertilization. Based on its functional role, we renamed this gene fertilization influencing membrane protein (Fimp). We discovered a new factor responsible for sperm-oocyte fusion in mammals, and this knowledge could be used to develop novel in vitro and in vivo infertility treatments as well as male contraceptives.
This article will be published in PNAS online, in the week of April 6.
Title: “Spermatozoa lacking Fertilization Influencing Membrane Protein (FIMP) fail to fuse with oocytes in mice”
Authors: Fujihara Y, Lu Y, Noda T, Oji A, Larasati T, Kojima-Kita K, Yu Z, Matzuk RM, Matzuk MM, and Ikawa M.
Links
-
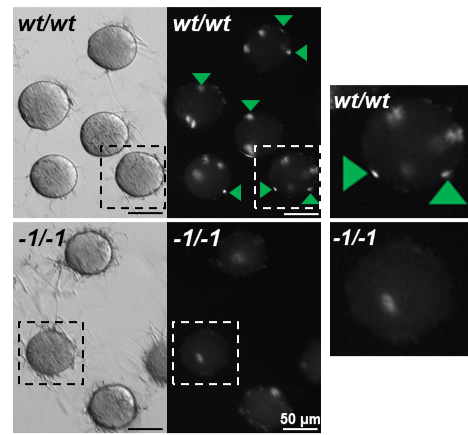
Fig. 1. Observation of sperm-oocyte fusion in wild-type and Fimp KO spermatozoa. Fimp KO (-1/-1) spermatozoa have impaired oocyte membrane fusion ability in vitro. Fused spermatozoa are indicated by green arrowheads. Enlarged images are indicated by dotted squares. Original image from PNAS (Fujihara et al.).
-
Fig. 2. Immunostaining of IZUMO1 in Fimp KO spermatozoa. IZUMO1 is used as a marker of the acrosome reaction. The acrosome reaction occurred in Fimp KO (-1/-1) spermatozoa. Acrosome reaction is determined by IZUMO1 localization (acrosome-intact spermatozoa: acrosomal cap pattern [orange dotted squares], acrosome-reacted spermatozoa: entire head pattern [green dotted squares]). Acrosome-reacted spermatozoa are indicated by asterisks. Original image from PNAS (Fujihara et al.).
-
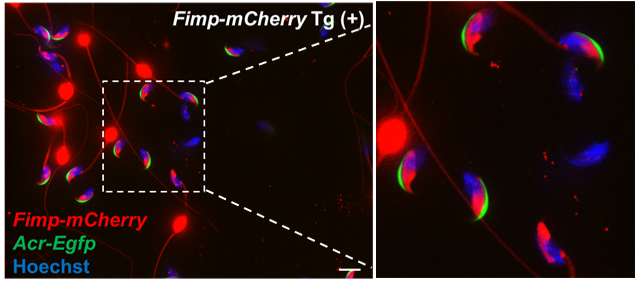
Fig. 3. Confocal microscopic observation of Fimp-mCherry Tg mouse spermatozoa. The TM form of FIMP and mCherry fused protein (red signals) were localized to the equatorial segment of the sperm head in acrosome-intact spermatozoa (Acr-EGFP [green signals] positive). However, after the acrosome reaction (Acr-EGFP [green signals] negative), both fluorescent and nonfluorescent spermatozoa were observed. Original image from PNAS (Fujihara et al.).
- Home
- Achievement
- Research Activities
- Discovery of sperm membrane protein FIMP involved in membrane fusion during fertilization (Ikawa Lab, in PNAS)