Elucidating the onset principle of fibrosis (Akira lab, in Immunity)
March 18, 2020
Research
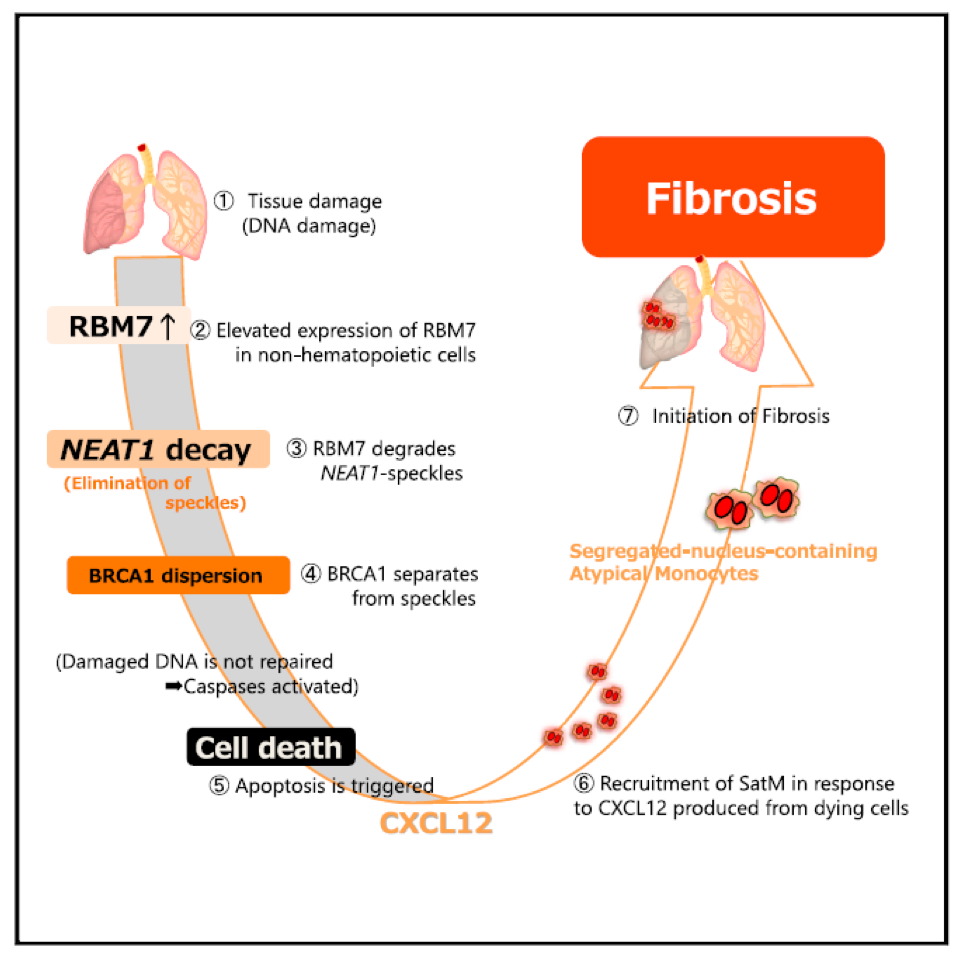
Fibrosis is an incurable disorder of unknown etiology. Segregated-nucleus-containing atypical monocytes (SatMs) discovered by the authors* are critical for the development of fibrosis. The research group of Kiyoharu Fukushima, Takashi Satoh and Shizuo Akira (Host Defense, IFReC, Osaka University) examined the mechanisms that recruit SatMs to pre-fibrotic areas. A screen based on cytokine expression in the fibrotic lung revealed that the chemokine Cxcl12, which is produced by apoptotic nonhematopoietic cells, was essential for SatM recruitment. Analyses of lung tissues at fibrosis onset showed increased expression of Rbm7, a component of the nuclear exosome targeting complex. Rbm7 deletion suppressed bleomycin-induced fibrosis and at a cellular level, suppressed apoptosis of nonhematopoietic cells. Mechanistically, Rbm7 bound to noncoding (nc) RNAs that form subnuclear bodies, including Neat1 speckles. Dysregulated expression of Rbm7 resulted in the nuclear degradation of Neat1 speckles, the dispersion of the DNA repair protein BRCA1, and the triggering of apoptosis. Thus, Rbm7 in epithelial cells plays a critical role in the development of fibrosis by regulating ncRNA decay and thereby the production of chemokines that recruit SatMs.
This article was published in Immunity on May 18, 2020
【Title】 ”Dysregulated expression of the nuclear exosome targeting complex component RBM7 in non-hematopoietic cells licenses the development of fibrosis”
【Authors】Kiyoharu Fukushima, Takashi Satoh, Fuminori Sugihara, Yuki Sato, Toru Okamoto, Yuichi Mitsui, Sachiyo Yoshio, Songling Li, Satoshi Nojima, Daisuke Motooka, Shota Nakamura, Hiroshi Kida, Daron M. Standley, Eiichi Morii, Tatsuya Kanto, Motoko Yanagita, Yoshiharu Matsuura, Takashi Nagasawa, Atsushi Kumanogoh, and Shizuo Akira
Related Links
>This article in Immunity
- Home
- NEWS&TOPICS







