A bacterial small RNA regulates the adaptation of H. pylori to the host environment (Mimuro Lab, in Nat Commun)
Long-term infection of the stomach with Helicobacter pylori can cause gastric cancer. However, the mechanisms by which the bacteria adapt to the stomach environment are poorly understood. Here, we show that a small non-coding RNA of H. pylori (HPnc4160, also known as IsoB or NikS) regulates the pathogen’s adaptation to the host environment as well as bacterial oncoprotein production. In a rodent model of H. pylori infection, the genomes of bacteria isolated from the stomach possess an increased number of T-repeats upstream of the HPnc4160-coding region, and this leads to reduced HPnc4160 expression. We use RNA- seq and iTRAQ analyses to identify eight targets of HPnc4160, including genes encoding outer membrane proteins and oncoprotein CagA. Mutant strains with HPnc4160 deficiency display increased colonization ability of the mouse stomach, in comparison with the wild-type strain. Furthermore, HPnc4160 expression is lower in clinical isolates from gastric cancer patients than in isolates derived from non-cancer patients, while the expression of HPnc4160’s targets is higher in the isolates from gastric cancer patients. Therefore, the small RNA HPnc4160 regulates H. pylori adaptation to the host environment and, potentially, gastric carcinogenesis.

Figure 2. Experimental method
Eight weeks after feeding mice and gerbils with H. pylori, we isolated H. pylori from the stomach and performed a whole-genome analysis.
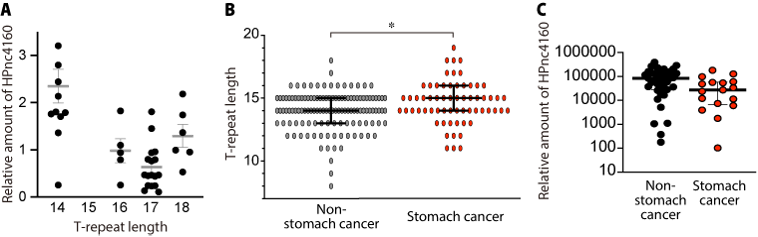
Figure 3. In the clinical isolates derived from gastric cancer patients, the continuous thymidine (T) sequence (T-repeat) upstream of HPnc4160 was longer than that of the clinical strains derived from non-gastric cancer patients, and the strains showed lower expression levels of HPnc4160.
H. pylori isolated from the stomach of Mongolian gerbil introduced a mutation that increases the T-repeat length in the upstream region of small RNA HPnc4160 (A). H. pylori in the stomach of patients with gastric cancer had a longer T-repeat length (B) and a lower amount of HPnc4160 (C) than H. pylori in the stomach of patients with non-gastric cancer.
This article was published in Nature Communications, on Feb 9th, 2021.
Title: “A bacterial small RNA regulates the adaptation of Helicobacter pylori to the host environment”
Authors: Ryo Kinoshita-Daitoku, Kotaro Kiga, Masatoshi Miyakoshi, Ryota Otsubo, Yoshitoshi Ogura, Takahito Sanada, Zhu Bo, Tuan Vo Phuoc, Tokuju Okano, Tamako Iida, Rui Yokomori, Eisuke Kuroda, Sayaka Hirukawa, Mototsugu Tanaka, Arpana Sood, Phawinee Subsomwong, Hiroshi Ashida, Tran Thanh Binh, Lam Tung Nguyen, Khien Vu Van, Dang Quy Dung Ho, Kenta Nakai, Toshihiko Suzuki, Yoshio Yamaoka, Tetsuya Hayashi, and Hitomi Mimuro.
Links
-
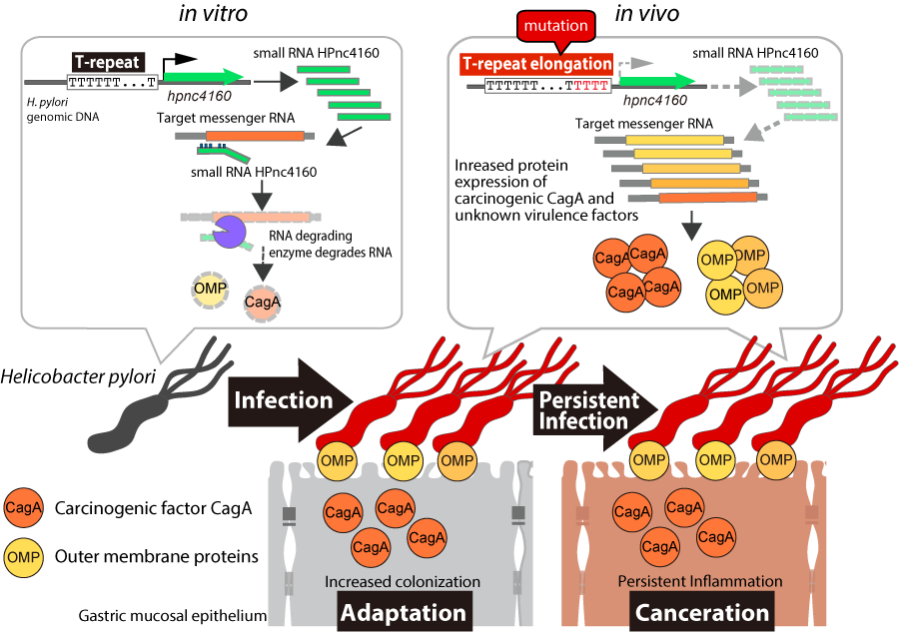
Figure 1. Persistent infection and canceration regulated by Helicobacter pylori small RNA HPnc4160 H. pylori's small RNA HPnc4160 suppresses the expression of messenger RNAs of virulence factors. When H. pylori infects gastric mucosa, a gene mutation is introduced into a continuous thymidine (T) sequence (T-repeat) located upstream of the genome encoding HPnc4160. Then, since the amount of HPnc4160 decreases, the amount of virulence factor protein increases, and H. pylori easily infects the gastric mucosa for a long period of time.
- Home
- Achievement
- Research Activities
- A bacterial small RNA regulates the adaptation of H. pylori to the host environment (Mimuro Lab, in Nat Commun)