CDCP1 promotes compensatory renal growth by integrating Src and Met signaling (Okada-Lab, in Life Science Alliance)
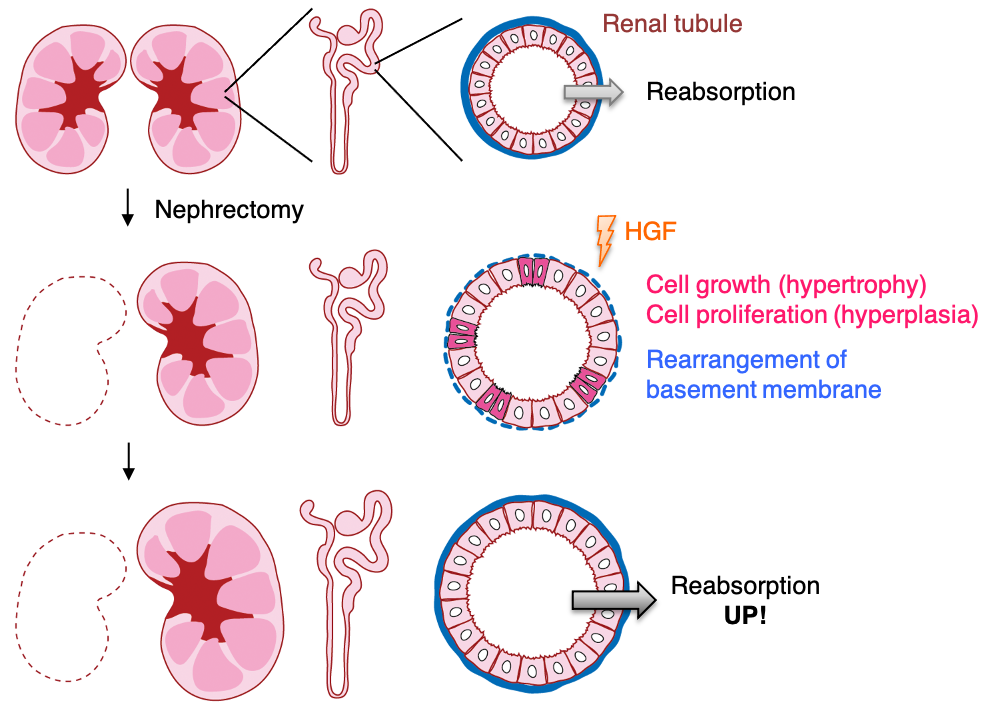
Controlling organ size during development and/or regenerative growth is important for maintaining organ function, body homeostasis, and health. The kidneys are paired organs that generate urine through the filtration of blood and reabsorption of water and nutrients, and kidney mass is strictly correlated with total body mass. The renal tubules constitute most of the mass and function, and have a remarkable capacity to undergo regenerative growth. Unilateral nephrectomy (UNX), a surgical procedure to reduce kidney mass, increases fluid flow in the remaining kidney, and promotes the subsequent growth/hypertrophy and proliferation/hyperplasia of tubular epithelial cells to compensate for the increased flow (Fig. 1). This compensatory renal growth is regulated by the activation of mechanistic target of rapamycin (mTOR) signaling pathways. However, interfering with the function of this pathway does not completely suppress renal growth, suggesting the potential contribution of one or more additional signaling pathways.
Compensatory renal growth also requires a number of growth factors, among which hepatocyte growth factor (HGF) plays an important role. HGF is produced by the surrounding or distal mesenchyme in the remaining kidney immediately after UNX, inducing the upregulation of its receptor, Met, in the renal tubules. However, the most important pathway for HGF-induced compensatory renal growth remains undefined.
The Madin-Darby canine kidney (MDCK) cell line was derived from renal tubule epithelial cells and is a physiologically relevant in vitro model to study the regulation of HGF functions in the kidney. When grown in three-dimensional cultures, MDCK cells spontaneously form spherical cysts that resemble renal tubules, comprising an epithelial monolayer and lumen. Upon stimulation with HGF, MDCK cysts undergo morphological alterations and form branched tubular structures.
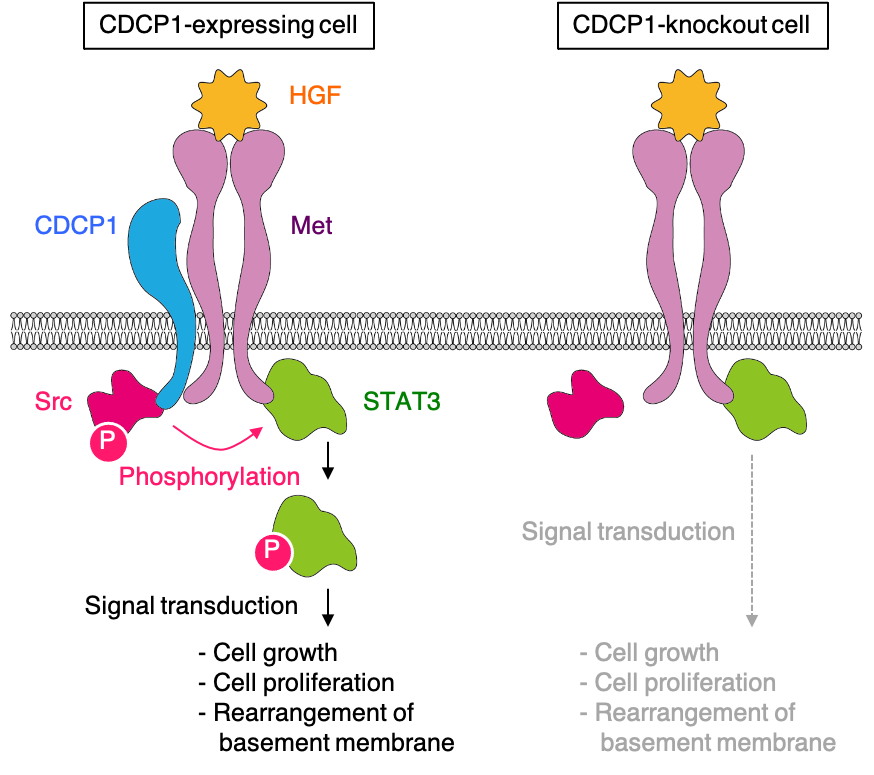
In this study, using three-dimensional cultures of MDCK cysts as a structural model of renal tubules, we identified Src and its membrane scaffold protein, CUB domain-containing protein 1 (CDCP1), as regulatory elements of HGF signaling. We then investigated the mechanisms underlying the functions of the CDCP1-Src axis in MDCK cysts and verified its physiological roles in compensatory renal growth using Cdcp1-knockout mice. The results obtained in this study demonstrates that CDCP1 plays a significant role in controlling HGF-induced compensatory renal growth by focally and temporally integrating Src and Met-STAT3 signaling (Fig. 2) and that transient degradation of basement membrane surrounding renal tubules is required for compensatory growth (Fig. 1).
This article was published online in Life Science Alliance, on Feb. 11, 2021.
Title: “CDCP1 promotes compensatory renal growth by integrating Src and Met signaling”
Authors: Kentaro Kajiwara, Shotaro Yamano, Kazuhiro Aoki, Daisuke Okuzaki, Kunio Matsumoto and Masato Okada
Links
- Home
- Achievement
- Research Activities
- CDCP1 promotes compensatory renal growth by integrating Src and Met signaling (Okada-Lab, in Life Science Alliance)