Structural basis for the Mg2+ recognition and regulation of the CorC Mg2+ transporter (Miki Lab, in Science Advances)
The CNNM/CorC family proteins are Mg2+ transporters that are widely distributed in all domains of life. In bacteria, CorC has been implicated in the survival of pathogenic microorganisms. In humans, CNNM proteins are involved in various biological events, such as body absorption/reabsorption of Mg2+ and genetic disorders.
Here, we determined the crystal structure of the Mg2+-bound CorC TM domain dimer. Each protomer possesses a single Mg2+ binding site with a fully dehydrated Mg2+ ion. The residues at the Mg2+ binding site are strictly conserved in both human CNNM2 and CNNM4, and many of these residues are associated with genetic diseases.
Furthermore, we determined the structures of the CorC cytoplasmic region containing its regulatory ATP binding domain. A combination of structural and functional analyses not only revealed the potential interface between the TM and cytoplasmic domains but also showed that ATP binding is important for the Mg2+ export activity of CorC.
This article was published in Science Advances on Feb. 10, 2021.
Title: “Structural basis for the Mg2+ recognition and regulation of the CorC Mg2+ transporter”
Authors: Yichen Huang†, Fei Jin†, Yosuke Funato†, Zhijian Xu, Weiliang Zhu, Jing Wang, Minxuan Sun, Yimeng Zhao, Ye Yu, Hiroaki Miki*, and Motoyuki Hattori* (†equal contribution, *corresponding authors)
Links
-
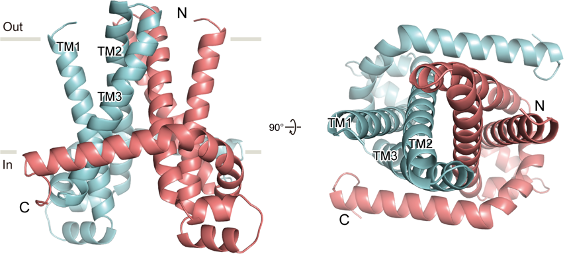
Fig.1 Cartoon representations of the Thermus parvatiensis CorC TM domain dimer in the Mg2+-bound form, viewed parallel to the membrane (left), from the extracellular side (right)
-
Fig.2 Close-up views of the Thermus parvatiensis CorC inter-subunit interface near the Mg2+-binding site. The overall structure is also shown. Amino acid residues are shown in stick representation, and Mg2+ ions are shown as green spheres.
- Home
- Achievement
- Research Activities
- Structural basis for the Mg2+ recognition and regulation of the CorC Mg2+ transporter (Miki Lab, in Science Advances)