J Cell Biol 2016 Nov 23 DOI: 10.1083/jcb.2016051212016/11/23
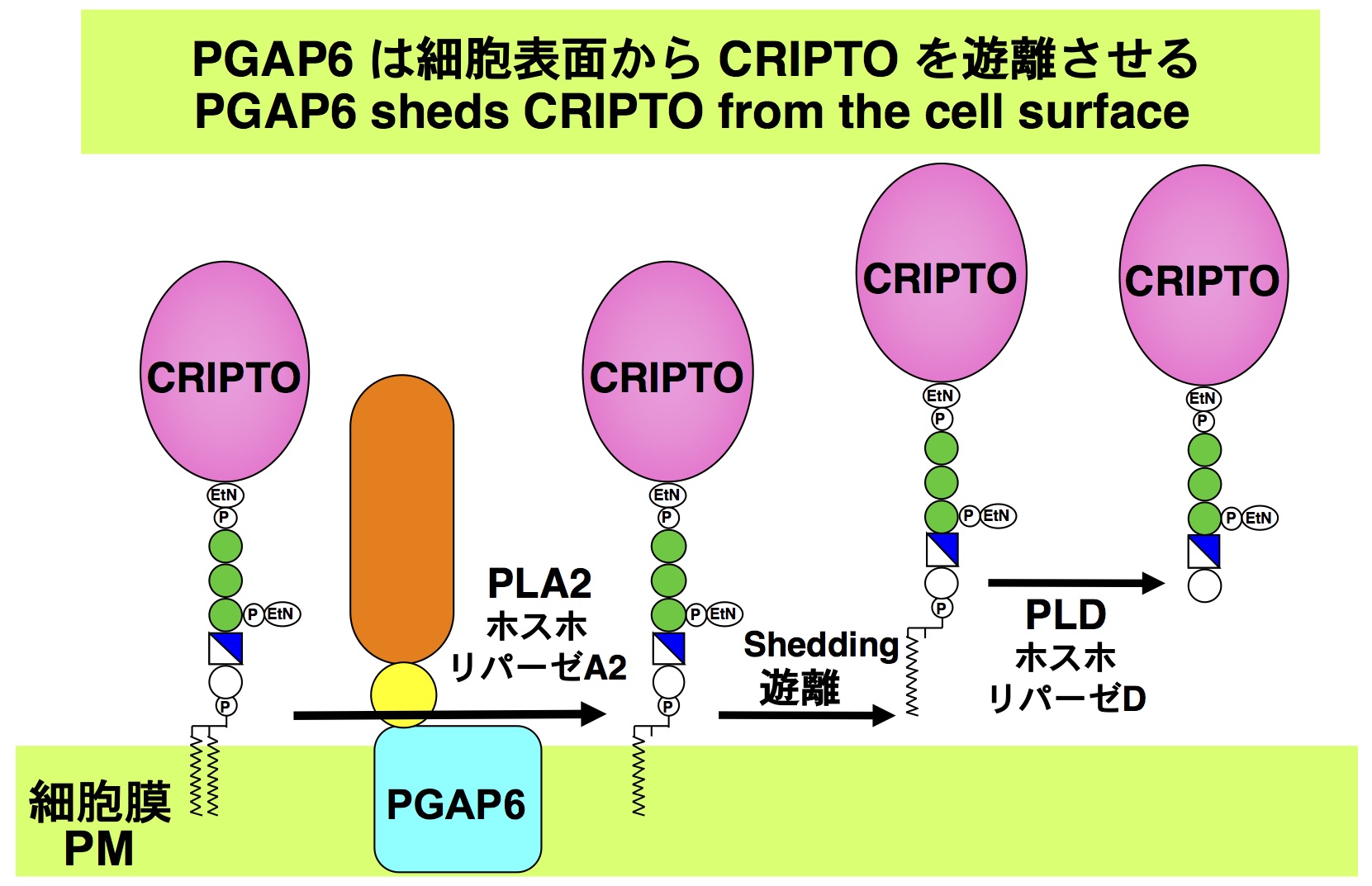
At least 150 human proteins are anchored to the plasma membrane by glycosylphosphatidylinositol (GPI). GPI-anchored proteins (GPI-APs) can be shed from the cell surface by GPI cleavage. In this study, we report a novel GPI-processing enzyme, termed PGAP6, which is a GPI-specific phospholipase A2 mainly localized at the cell surface. CRIPTO, a GPI-AP, which plays critical roles in early embryonic development by acting as a Nodal co-receptor, is a highly sensitive substrate of PGAP6. CRIPTO shed by PGAP6 was active as a co-receptor for Nodal. Homozygous PGAP6 knockout mice showed defects in early embryonic development, particularly in the formation of the anterior–posterior axis, which are common features with CRIPTO knockout embryos. These results suggest that PGAP6 plays a critical role in Nodal signaling through CRIPTO shedding.
Links
- Home
- Achievement
- Research Activities
- J Cell Biol 2016 Nov 23 DOI: 10.1083/jcb.2016051212016/11/23