Nat Microbiol doi:10.1038/nmicrobiol.2016.54 2016/04/25
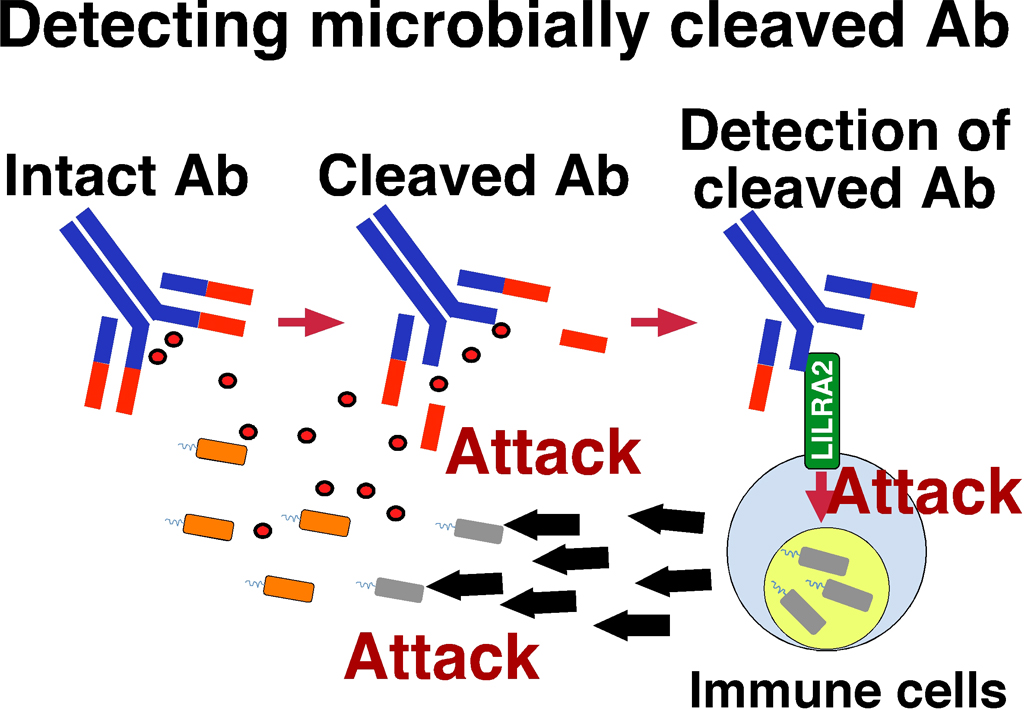
Microbial pathogens degrade immunoglobulin or antibody by producing proteases and thus disrupt antibody function for host defense against pathogens. However, it has remained largely unknown how the host responds to microbially degraded products. Here, we found that antibodies disrupted by Mycoplasma hyorhinis are specifically detected by leukocyte immunoglobulin-like receptor A2 (LILRA2), an orphan activating receptor expressed on human myeloid cells. Proteases from Legionella pneumophila , Streptococcus pneumonia and Candida albicans also cleaved antibodies. Moreover, stimulation of primary monocytes via LILRA2 inhibited the growth of Legionella pneumophila in primary monocytes. More importantly, cleaved immunoglobulins were detected in the patients with bacterial infections such as otitis media, infected atheroma and cellulitis, and stimulated LILRA2-expressing cells. Our findings demonstrate that LILRA2 is a type of innate immune receptors in the host immune system to detect immunoglobulin abnormalities caused by microbial pathogens. Therefore, controlling the LILRA2 function will be useful for the development of new therapy and vaccine against infectious diseases.
2016.5.31
Following links are review articles for this paper.
Nat Reviews Immunology
Nature Microbiology
Links
- Home
- Achievement
- Research Activities
- Nat Microbiol doi:10.1038/nmicrobiol.2016.54 2016/04/25