Arase Lab/Division of Host Defense Department of Immunochemistry
We have been studying interactions between pathogens and various paired receptors. In addition, we found that MHC class II molecules function as molecular chaperones to transport misfolded proteins to the cell surface. Analyses of misfolded proteins transported to the cell surface revealed that they are involved in autoimmune diseases by acting as a target for autoantibodies.
Interaction between immune receptors and pathogens
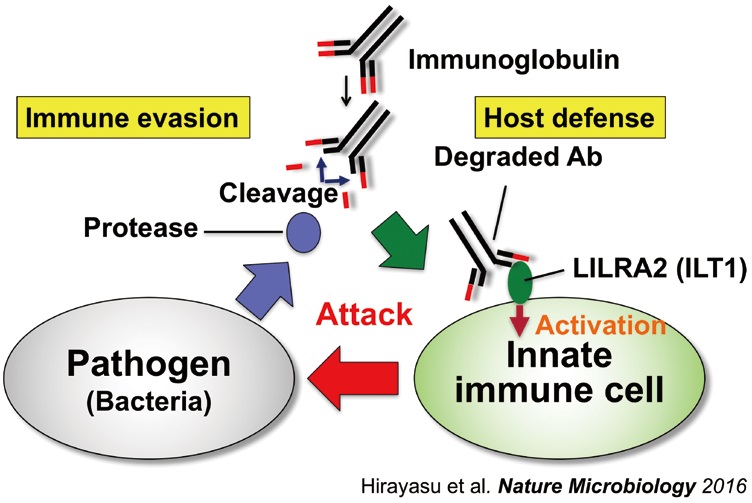
Immune cells express "paired" activating and inhibitory receptors that are highly homologous. The inhibitory receptors recognize self-antigens and downregulate immune response to the self. On the other hand, we found that some inhibitory receptors are used by pathogens for immune evasion (Fig. 1). By contrast, we found that LILRA2, an orphan activating receptor expressed on human myeloid cells, recognizes abnormal immunoglobulins cleaved by microbial proteases but not normal immunoglobulins. Because immunoglobulins are important for host defense, their degradation is very dangerous in terms of immunity (Fig. 2). In this way, paired receptors play an important role not only in immune regulation but also in host defense against pathogens.
Misfolded proteins complexed with MHC class II molecules trigger autoimmune disease
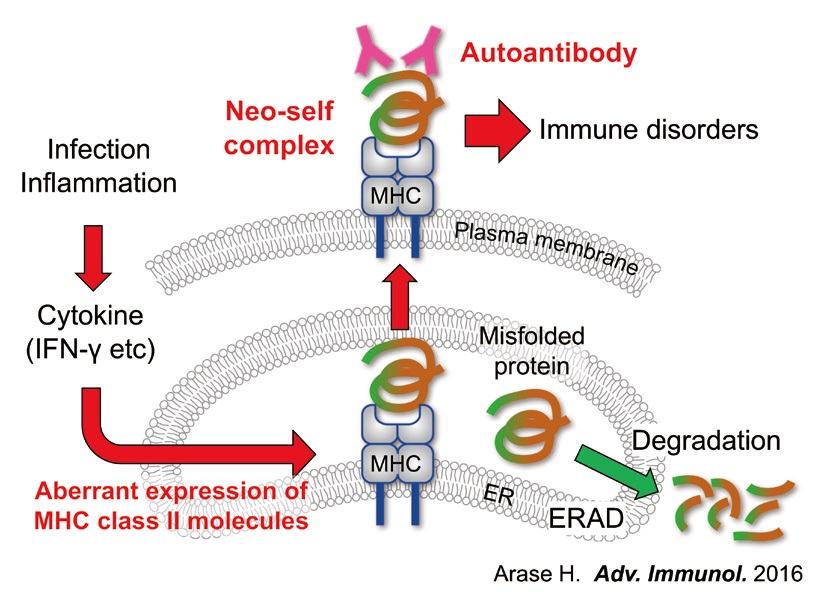
Allelic polymorphisms in MHC class II molecules are strongly associated with susceptibility to many autoimmune diseases. However, it is unclear how MHC class II molecules are involved in autoimmune disease susceptibility. We found that misfolded cellular autoantigens are rescued from protein degradation by MHC class II molecules. Furthermore, we found that misfolded proteins complex with MHC class II molecules and become targets for autoantibodies.
Autoantibody binding to misfolded proteins that are transported to the cell surface by MHC class II molecules correlated strongly with susceptibility to autoimmune disease, suggesting that misfolded proteins, which normally would not be presented to the immune system, can be targets for autoantibodies by acting as “neo self” antigens, which are involved in the pathogenicity of autoimmune diseases (Fig.3).
-
Fig. 1. Inhibitory receptors play an important role in immune regulation, whereas pathogens exploit inhibitory receptors for immune evasion. We found malaria parasite has a mechanism to suppress the host immune response by using an inhibitory receptor, LILRB1, contributing to the pathogenesis of severe malaria.
-
Fig. 2. Activating paired receptors play a role in host defense against bacterial infection Activating paired receptor, LILRA2, recognizes immunoglobulin cleaved by bacterial protease activate innate immune cells (Hirayasu et al. Nat. Microbiol. 2016).
-
Fig. 3. Misfolded proteins complexed with MHC class II molecules are targets for autoantibodies. Misfolded cellular proteins are transported to the cell surface without being processed to peptides by associating with MHC class II molecules in the ER. Furthermore, misfolded proteins complexed with MHC class II molecules encoded by disease-susceptible alleles are specifically recognized by autoantibodies. This suggests that misfolded proteins complexed with MHC class II molecules are natural autoantigens for autoantibodies, which affect susceptibility to autoimmune diseases (Arase Adv. Immunol. 2016).
Staff
- Prof. : Hisashi Arase (concur.)
- Associ. Prof.: Jin Hui
- Asst. Prof: Shunsuke Mori
- SA Asst. Prof: Wataru Nakai
Website
Publications
(1) RIFINs displayed on malaria-infected erythrocytes bind KIR2DL1 and KIR2DS1. Sakoguchi et al., Nature (2025).
(2) Neoself-antigens are the primary target for autoreactive T cells in human lupus. Mori et al., Cell (2024) 187: 6071-6087.
(3) An infectivity-enhancing site on the SARS-CoV-2 spike protein targeted by antibodies. Liu et al., Cell (2021) 184:3452-3466.
(4) Immune evasion of Plasmodium falciparum by RIFIN via inhibitory receptors. Saito F., et al., Nature (2017) 552:101–105.
(5) PILRα is a herpes simplex virus-1 entry co-receptor that associates with glycoprotein B. Satoh T., et al., Cell (2008) 132:935-44.
- Home
- Laboratories
- Arase Lab