Akira Lab/Division of Host Defense Department of Host Defense
Innate immunity is a defense system triggered by pattern recognition receptors, which recognize various pathogens such as bacteria, fungi, and viruses, and induce the production of inflammatory factors to trigger immune responses. To gain comprehensive understanding of the molecular mechanisms responsible for innate immunity in vivo, our lab focuses on the following theme:
Exploration of the relationship between immune responses and mechanisms that ensure mRNA stability
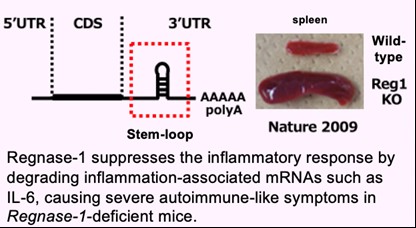
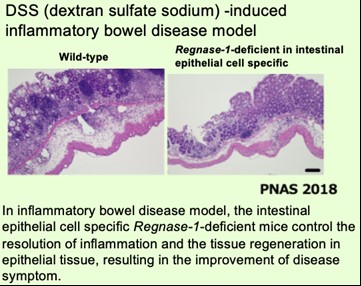
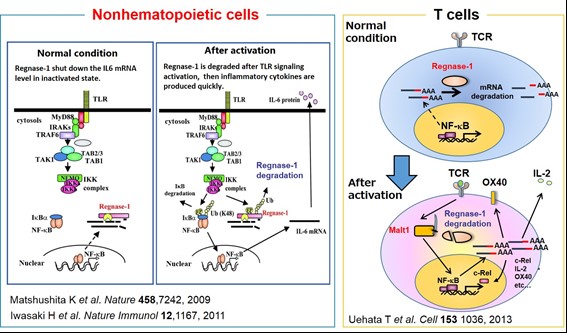
We have been investigating the comprehensive innate immune response induced by pattern recognition receptors that sense various pathogens. By studying the Toll-like receptor (TLR)-signaling pathway we found that a novel regulatory mechanism, “mRNA stability,” controls inflammatory responses. Regnase-1, an endoribonuclease, constitutively degrades mRNAs encoding inflammatory cytokines. Once the TLR pathway is activated, the synthesis of mRNAs encoding inflammatory cytokines is promptly induced along with reduced enzymatic activity of Regnase-1. As a result, mRNAs encoding inflammatory cytokines are stably expressed, resulting in an ongoing inflammatory response. Thus, endogenous Regnase-1 negatively regulates the stability of mRNAs encoding inflammatory cytokines in normal immune cells. Once pathogens invade cells, Regnase-1-mediated negative regulation is released, leading to inflammation. The temporary inactivation of Reganse-1 upon cellular activation is also found in stimulation with other proinflammatory cytokines or T-cell activation, suggesting the importance of Reganse-1 protein modification for regulation of innate immune response. Furthermore, we have found that Regnase-1 not only regulates inflammation and immune activation but also plays a critical role in tissue homeostasis through the mRNA degradation. To understand the novel aspects of mRNA regulation by Regnase-1, we will identify the Reganse-1 target genes associated with RNA metabolism in immune and non-immune cells.
Staff
- SA Prof. : Shizuo Akira (concur.)
- SA Assoc. Prof.: Kazuhiko Maeda (concur.)
- SA Asst. Prof.: Akihiko Murata(concur.)
Website
Publications
(1) Dysregulated expression of the nuclear exosome targeting complex component Rbm7 in nonhematopoietic cells licences the development of Fibrosis. Fukushima et al. Immunity. (2020) 52(3): 542-556.
(2) Phosphorylation-dependent Regnase-1 release from endoplasmic reticulum is critical in IL-17 response. Tanaka et al. J. Exp. Med. (2019) 216(6): 1431-1449.
(3) Regnase-1 controls colon epithelial regeneration via regulation of mTOR and purine metabolism. Nagahama et al. Proc Natl Acad Sci U S A. (2018) 115(43): 11036-11041.
(4) Identification of an atypical monocyte and committed progenitor involved in fibrosis. Satoh et al. Nature. (2017) 541(7635): 96-101
(5) Malt1-Induced Cleavage of Regnase-1 in CD4+ Helper T Cells Regulates Immune Activation. Uehata et al. Cell. (2013) 153(5):1036-1049.
(6) Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Matsushita et al. Nature. (2009) 458(7242):1185- 1190.
- Home
- Laboratories
- Akira Lab