Tsukamoto Lab/International Research Center for Infectious Diseases Laboratory of Bacterial Zoonoses
Zoonoses are infectious diseases caused by pathogens, including bacteria, viruses, and parasites, transmitted between vertebrate animals and humans. Among the zoonotic pathogens, some exhibit no virulence in animals but can cause diseases in humans. What factors give rise to such host specificity? Reaching a conclusive answer is not straightforward because of the variety of factors among pathogens and the intricate interplay of host factors. Our research group focuses on zoonotic bacteria, aiming to uncover the molecular mechanisms of their pathogenicity and symbiosis. Through our studies, we aim to unravel the enigma of host specificity associated with these bacteria.
Understanding the mechanism of Bartonella infection and pathogenesis
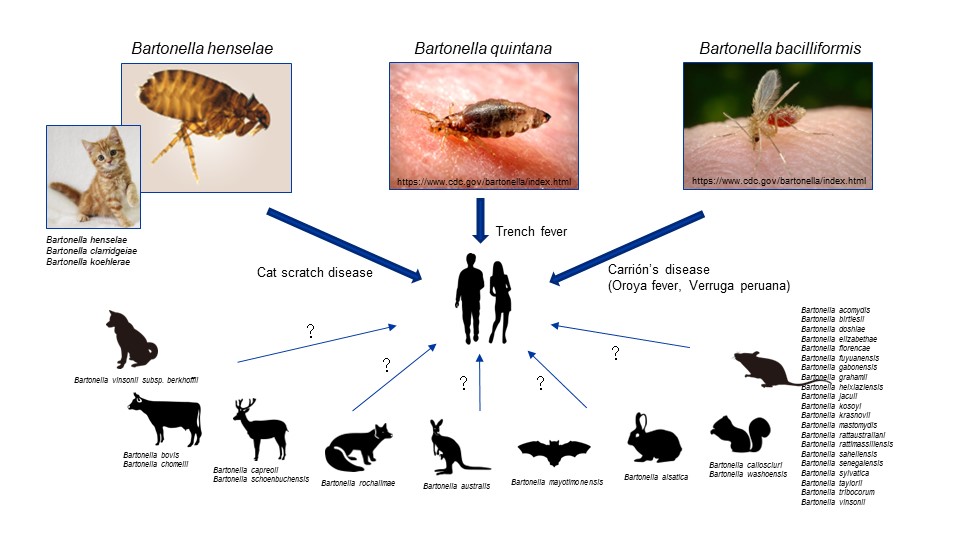
Bacteria classified under the genus Bartonella inhabit a variety of mammals and blood-sucking arthropods, and over 40 species have been identified to date. Among them, the most well-known is Bartonella henselae, the causative agent of cat-scratch disease. As a natural reservoir host, this microbe primarily resides in cats, with carrier cats remaining asymptomatic; however, human infection results in lymphadenopathy with fever. Some species other than B. henselae are pathogenic in humans. We are exploring the factors associated with the infectivity and pathogenicity of these various Bartonella species. By using human cells and tissue models, we are making progress with elucidation of the molecular mechanisms involved in disease initiation.
Angiogenic factor produced by Bartonella
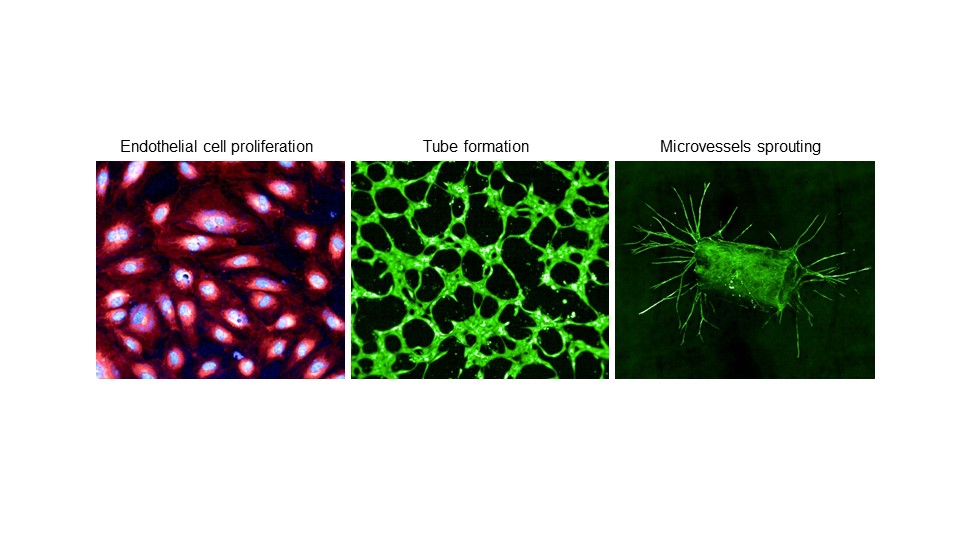
Upon infecting humans, Bartonella targets and invades endothelial cells. Additionally, this bacterium stimulates angiogenesis by promoting the growth of endothelial cells. In other words, Bartonella has the inherent ability to stimulate the proliferation of endothelial cells, which serve as the replicative niche, potentially contributing to the establishment of infection and the manifestation of pathogenicity. We have identified a protein named BafA, which is released by the bacteria and is directly involved in inducing angiogenesis. BafA interacts with vascular endothelial growth factor receptor, which is located on the surface of human endothelial cells, leading to endothelial cell proliferation and angiogenesis. We are currently analyzing the molecular structure and mechanism of action of BafA, with the aim of elucidating how it is implicated in bacterial infectivity and pathogenicity.
-
Bacteria of the genus Bartonella are harbored in various animals and blood-sucking arthropods. Many of these bacteria are suspected to be responsible for zoonotic diseases, and three specific species, Bartonella henselae, Bartonella quintana, and Bartonella bacilliformis, are widely known to cause human infections.
-
Effect of angiogenic factor BafA produced by Bartonella. BafA enhances proliferation of vascular endothelial cells (left), promotes tubular structure formation (middle), and causes sprouting of microvessels from the aortic ring (right).
Staff
- SA Assoc. Prof.: Kentaro Tsukamoto
Website
Publications
(1) Differential vasoproliferative traits of Bartonella henselae strains associated with autotransporter BafA variants. Kondo, Y. et al., Microbiol Spectr (2025) 13, e0192524
(2) The autotransporter BafA contributes to the proangiogenic potential of Bartonella elizabethae. Suzuki, N., et al., Microbiol Immunol (2023) 67, 248-257
(3) Comparison of biological activities of BafA family autotransporters within Bartonella species derived from cats and rodents. Kumadaki, K. et al., Infect Immun (2023) 91, e0018622
(4) The passenger domain of Bartonella bacilliformis BafA promotes endothelial cell angiogenesis via the VEGF receptor signaling pathway. Tsukamoto, K. et al., mSphere (2022) 7, e0008122
(5) The Bartonella autotransporter BafA activates the host VEGF pathway to drive angiogenesis. Tsukamoto, K. et al., (2020) Nat Commun 11, 3571
- Home
- Laboratories
- Tsukamoto Lab