Kotani Lab/Division of Cellular and Molecular Biology Department of Regulation of Infectious Cancer
Basic research to develop novel therapies for refractory infectious and hematopoietic tumors.
From the new perspectives of extracellular vesicles, non-coding RNAs, lipids, and the tumor microenvironment, we are conducting basic research studies to develop novel therapies for infectious tumors, especially Epstein-Barr virus (EBV)-associated lymphomas, and refractory hematopoietic malignancies.
Since EBV-associated cancers are rare in Europe and the United States, but common in East Asia, it is desirable to promote research in this area in Japan. In other words, unless this research is promoted in Japan, the prognosis of patients with EBV-associated lymphomas cannot be expected to improve, because of global ignorance.
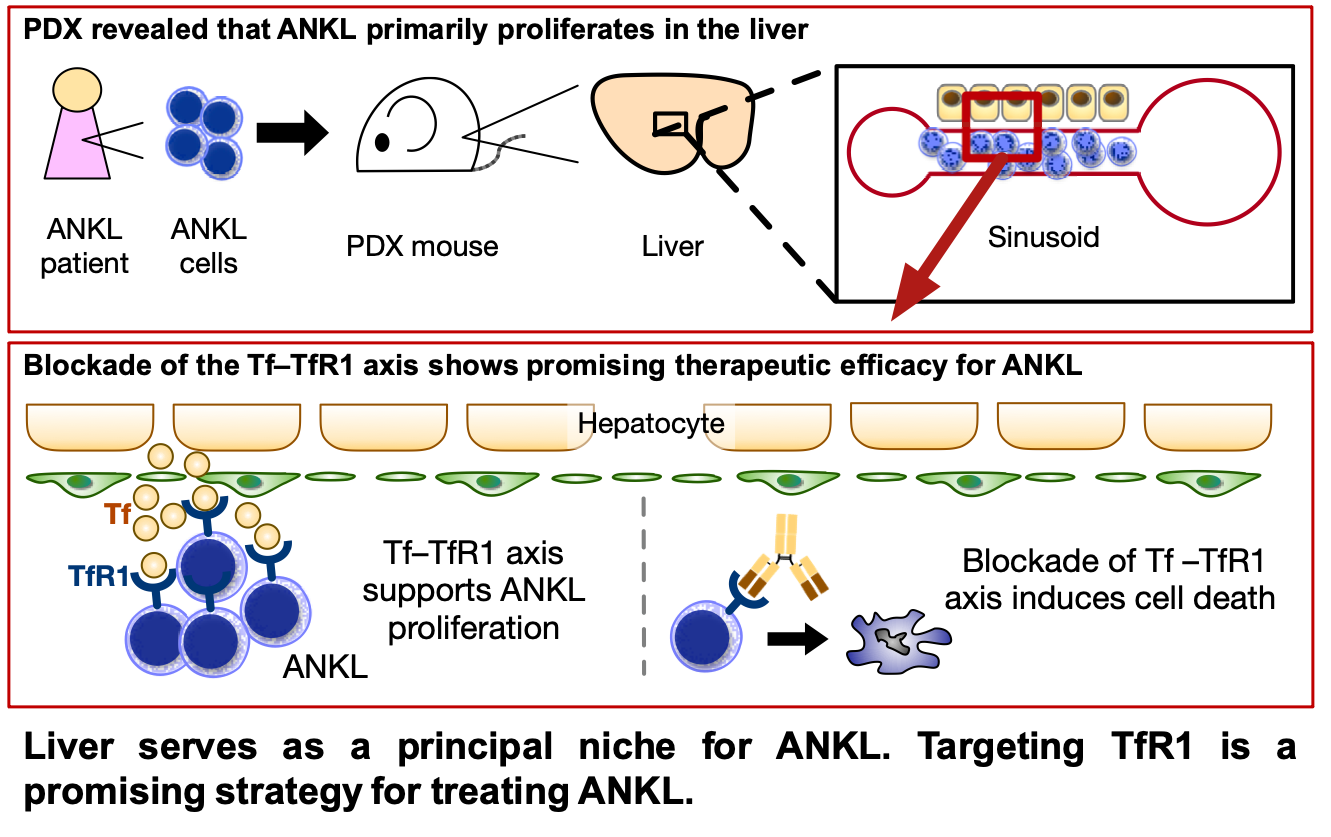
Considering the current knowledge on these diseases, we have been working with a sense of mission on the identification of therapeutic targets in EBV-associated lymphomas. Notably, based on our results already been published (Blood 2023), the clinical trial has been started in 2023, implying that our research results are being used for the benefit of humans (Figure 1).
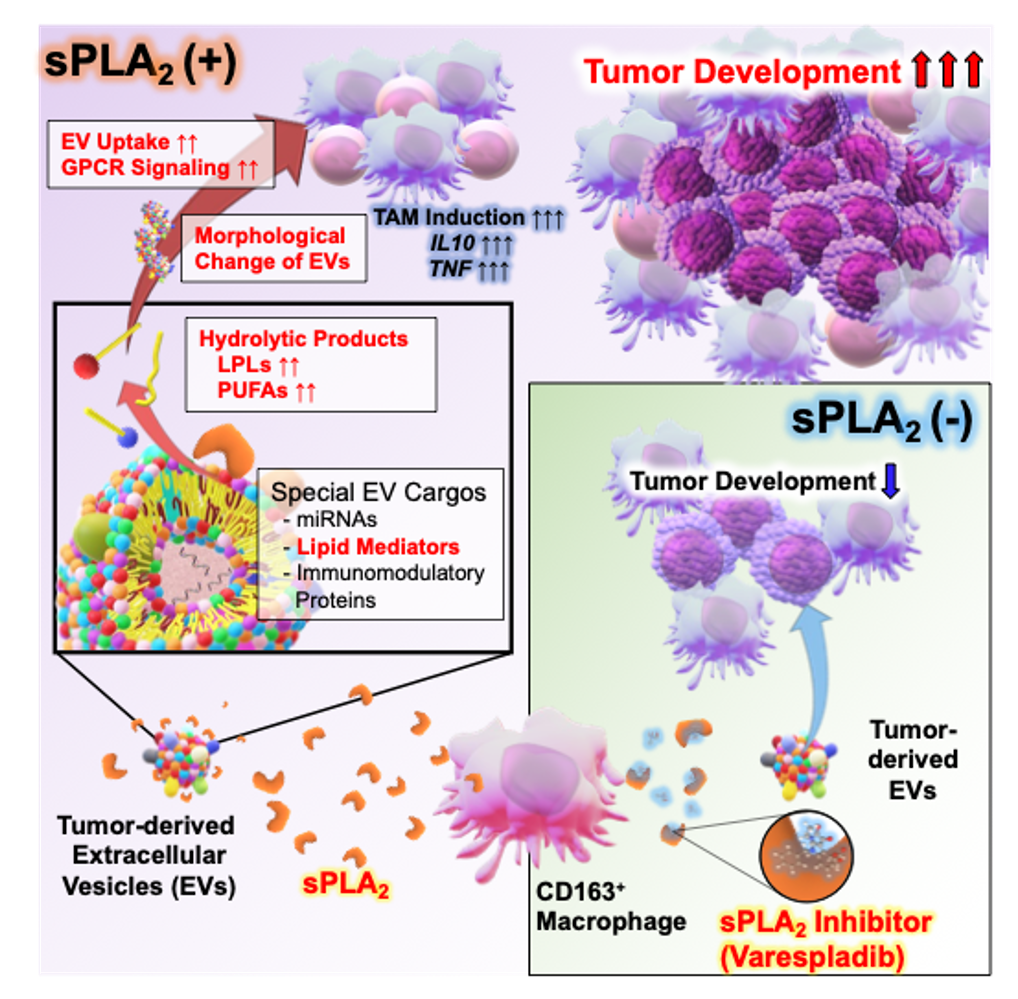
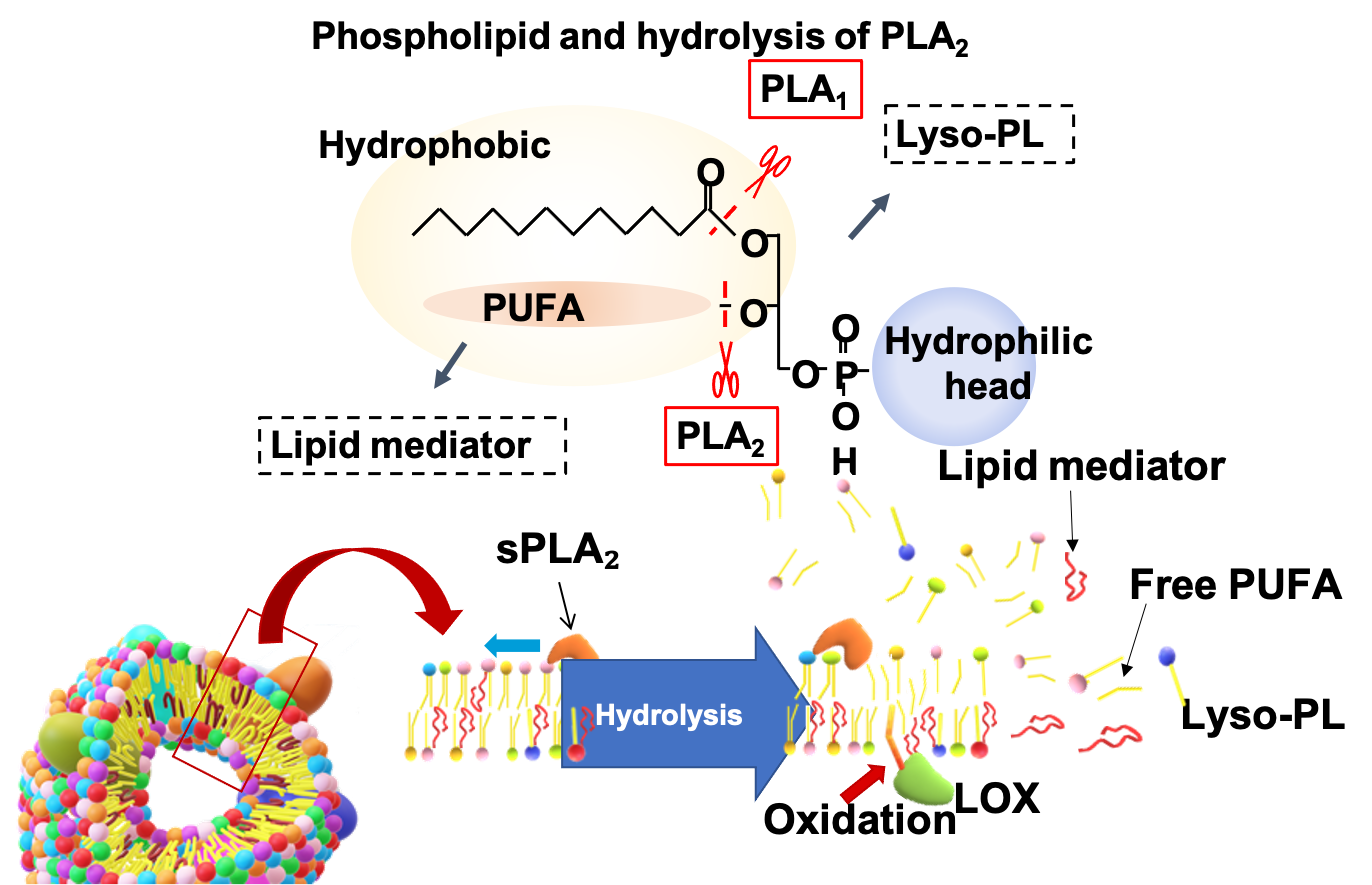
Besides, due to the infection process involved, viral cancers develop in a short period of time, originating extreme biological conditions within cancer cells. The mechanisms underlying these physiological conditions can be reveled and analyzed in detail, leading to the construction of new scientific knowledge. For example, novel non-coding RNAs and the non-canonical mechanical function of extracellular vesicles were discovered while studying EBV-associated cancers (NAR 2017, Cell Metabolism 2022) (Fig. 2).
We aim to continue this research for the identification of therapeutic targets in EBV-associated cancers and for generating new scientific knowledge.
In addition, the research line involving hepatitis B virus (HBV), which was initiated to apply the knowledge gained from studying EBV-associated diseases into other viral diseases, has led us to expand our research targets to include liver disease, intestinal disease, and cytokine syndrome. Moreover, new technologies are being developed by integrating the results obtained from our work involving EBV and HBV (Figure 3).
Staff
- Prof.: Ai Kotani
- Assoc. Prof.: Masahiro Oka
- Asst. Prof.: Takeshi Kamakura
- SA Asst. Prof.: QIU HANTIAN
- SA Asst. Prof.: Kaku Goto
- JSPS Postdoc.: Akane Kanamori
- Postdoc.: LI XIAODONG
Website
Publications
1. Amino acid influx via LAT1 regulates iron demand and sensitivity to PPMX-T003 of aggressive natural killer cell leukemia. Yanagiya R. et al. Leukemia (2024) 38(8):1731-1741.
2. Hepatic niche leads to aggressive NK-cell leukemia proliferation through transferrin-transferrin receptor 1 axis. Kameda K., et al., BLOOD (2023) 142(4):352-364
3. Secreted phospholipase A2 modifies extracellular vesicles and accelerates B cell lymphoma. Kudo K., et al., Cell Metab, (2022) 34, 615-633.e8.
4. Anti-HBV drug entecavir ameliorates DSS-induced colitis through PD-L1 induction. Yamamoto Y., et al., Pharmacol Res (2022) 179, 105918.
5. Human hepatocyte-derived extracellular vesicles attenuate the carbon tetrachloride-induced acute liver injury in mice. Kakizaki M., et al., Cell Death and Disease (2021) 12, 1010.
6. PD-L1/L2 protein levels rapidly increase on monocytes via trogocytosis from tumor cells in classical Hodgkin lymphoma. Kawashima M., et al., Leukemia (2020) 34(9):2405-2417.
7. Role of exosomes as a proinflammatory mediator in the development of EBV-associated lymphoma. Higuchi H., et al., BLOOD (2018) 131, 2552-2567.
8. Novel functional small RNAs are selectively loaded onto mammalian Ago1. Yamakawa N., et al., Nucleic acids research (2014) 42(8), 5289-301.
9. MicroRNA-126-mediated control of cell fate in B-cell myeloid progenitors as a potential alternative to transcriptional factors. Okuyama K., et al., PNAS (2013) 110(33) 13410-13415.
10. Imatinib mesylate directly impairs class switch recombination through down-regulation of AID: its potential efficacy as an AID suppressor. Kawamata T., et al., BLOOD (2012) 119(13), 3123-3127.
- Home
- Laboratories
- Kotani Lab