/Division of Host Defense Laboratory of Immunometabolism
The interconnection of multiple organs maintains our health. When an infection occurs, the immune system and other organs cooperate to eliminate the pathogen. In our laboratory, we focus on metabolism and immune cells. We study (1) the role of metabolic signals in immune cells, (2) the influence of immune cells on systemic metabolism, and (3) the role of metabolic signals in various cells.
Exploring new physiological roles of macrophages
Macrophages function in the normal development of the fetus and are involved in processing dead cells and tissue repair in the adult body. As a host defense function, macrophages use pattern receptors to detect the invasion of foreign microorganisms and phagocytose pathogenic microorganisms such as bacteria. It can also secrete cytokines to recruit various immune cells to the site of infection and induce inflammation.
Recently, it has been pointed out that macrophages may promote disease in several pathological conditions, such as diabetes, organ fibrosis, and cancer progression. In addition, macrophages have been reported to be involved in heat production in brown adipose tissue and conduction in the atrioventricular node as non-canonical functions. Our laboratory is working to elucidate the unknown functions of macrophages.
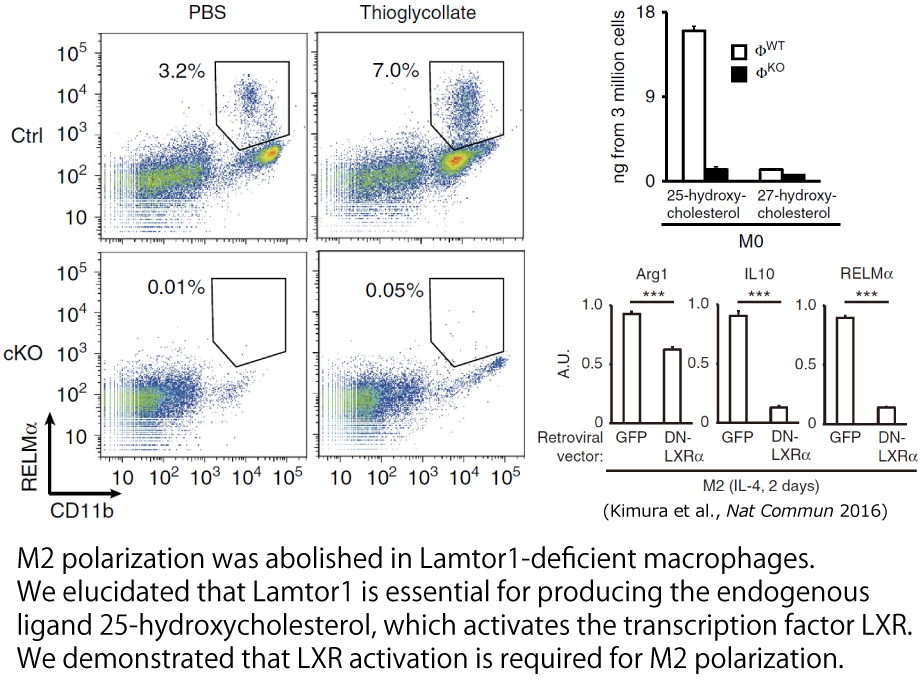
Exploring new functions of the metabolic signaling pathway located at the lysosome
We are investigating the functions of metabolic signals at lysosomal membranes, starting with p18(Lamtor1), a protein discovered in Research Institute for Microbial Diseases. p18 forms a heteropentamer complex, Ragulator, with other proteins and is a scaffold for amino acid-induced mTORC1 activation.
Various nutritional signals funnel to activate the kinase complex mTORC1, which, in turn, activates multiple downstream signals involved in cell proliferation and protein synthesis. Therefore, mTORC1 acts as a metabolic hub. However, the mechanism by which mTORC1 regulates specific downstream signals was unknown. Our group has elucidated that Ragulator is essential as a substrate-specific scaffold for mTORC1 phosphorylating TFEB. We will continue to elucidate the function of Ragulator.
Staff
- Prof.: Tetsuya Iida (concurr.)
- SA Asst. Prof.: Tetsuya Kimura
Publications
- (1) The Ragulator complex serves as a substrate-specific mTORC1 scaffold in regulating the nuclear translocation of transcription factor EB. Kimura T., et al., J Biol Chem. (2022) 298:101744
(2) The lysosomal Ragulator complex plays an essential role in leukocyte trafficking by activating myosin II. Nakatani T., et al., Nat Commun. (2021) 12:3333
(3) p18/Lamtor1-mTORC1 signaling controls development of mucin-producing goblet cells in the intestine. Ito S., et al., Cell Struct Funct. (2020) 45:93-105.
(4) Semaphorin 6D reverse signaling controls macrophage lipid metabolism and anti-inflammatory polarization. Kang S., et al., Nat Immunol. (2018) 19:561-570.
(5) Lysosomal protein Lamtor1 controls innate immune responses via nuclear translocation of transcription factor EB. Hayama Y., et al., J Immunol (2018) 200:3790-3800.
(6) Lamtor1 is critically required for CD4+ T cell proliferation and regulatory T cell suppressive function. Hosokawa T., et al., J Immunol (2017) 199:2008-2019.
(7) Polarization of M2 macrophages requires Lamtor1 that integrates cytokine and amino-acid signals. Kimura T., et al., Nat Commun (2016) 7:13130