Watanabe Lab/Division of Infectious Disease Department of Molecular Virology
A virus is a simple and very tiny structure composed of protein shells and nucleic acids. It is surprising that such a small entity can sometimes cause pandemics, resulting in significant global damage. In our laboratory, we focus on viruses that cause zoonotic diseases, such as influenza, COVID-19, and Ebola, and elucidate the mechanisms of host adaptation, replication, and pathogenicity of viruses. We also aim to develop safe and effective vaccines by utilizing the knowledge obtained from our fundamental research.
- Research on emerging zoonotic viruses
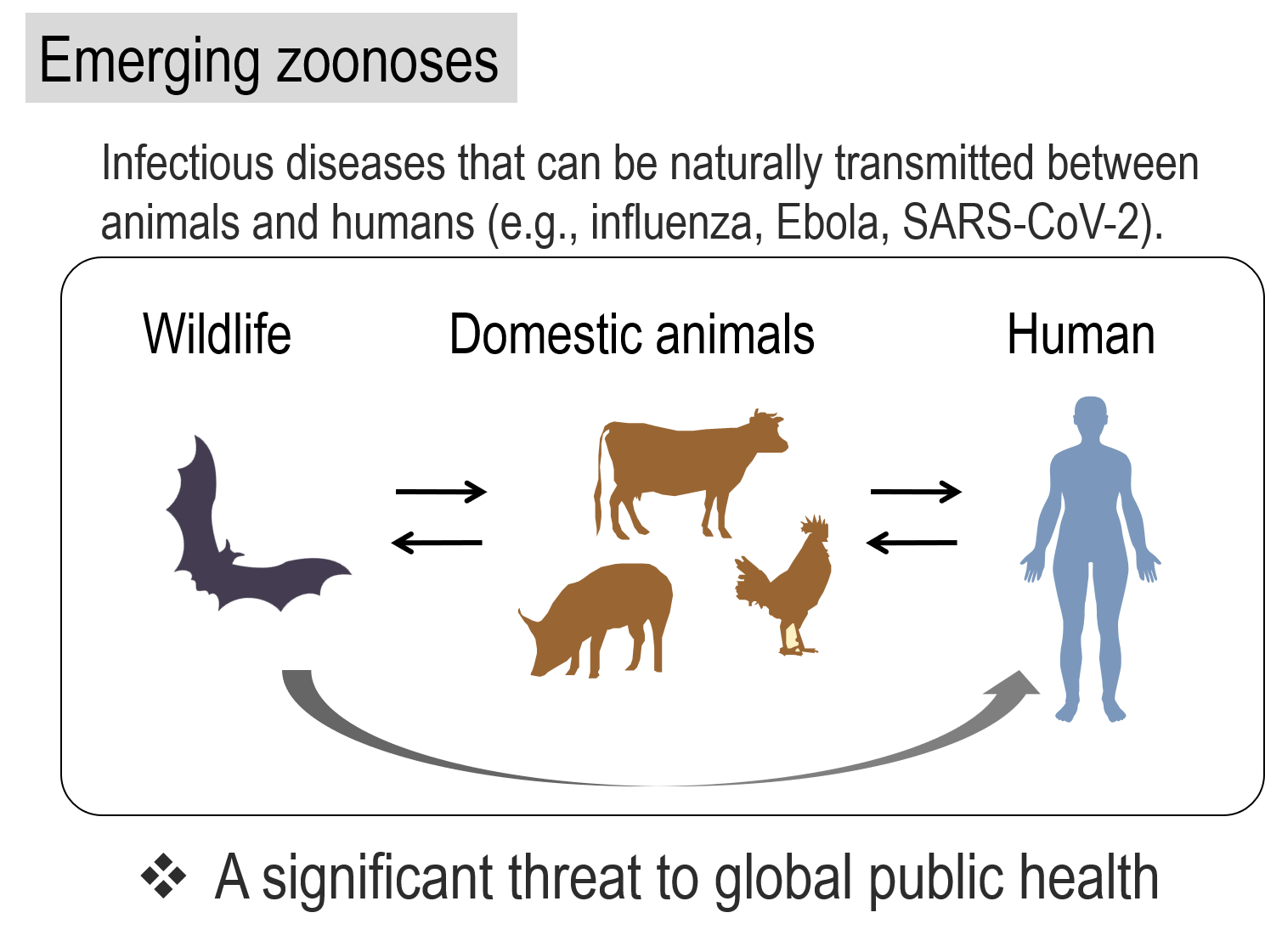
The development of modern medicine has allowed us to eliminate numerous infectious diseases; however, humans constantly face threats from novel infectious diseases that were previously unrecognized. These so-called emerging infectious diseases are caused by newly identified species or strains; for example, Ebola, AIDS, SARS, avian influenza, and MERS which have all appeared in human society over the last several decades. More recently, SARS-CoV-2 emerged in China at the end of 2019 and spread across the world, causing the COVID-19 pandemic. Most emerging infectious diseases are zoonotic and are caused by viruses that originate in wild animals. To cause zoonosis, the pathogens that originate in animals must cross the species barrier and transmit to humans. If these pathogens are able to efficiently transmit from human to human, a pandemic is likely to emerge and threaten the lives of humans around the world.
Influenza A viruses, one of the most important zoonotic viruses, cause annual epidemics and recurring pandemics. In addition, recent sporadic human infections with avian influenza viruses have raised concerns regarding the pandemic potential of these viruses. Although influenza A viruses can infect a wide range of species, host restriction usually constrains their interspecies transmission; however, mammalian-adaptive mutations have been identified in some viral proteins that allow avian influenza viruses to overcome the species barrier.
In our laboratory, we aim to elucidate the mechanisms of viral replication, pathogenicity, and the viral strategy to adapt mammalian hosts for influenza viruses. Further, we have been working with collaborators in Sierra Leone in West Africa and Brazil in South America to understand the epidemic of various zoonotic viruses in wild animals.
- Development of safe and effective vaccines for viral diseases
The current inactivated influenza vaccine has low immunogenicity. The use of adjuvants has been considered to enhance the vaccine effect, but there are still concerns about the safety of unwanted side effects. Given this and our desire to develop a safe and effective influenza vaccine, we are trying to find novel adjuvant candidates with superior safety and a robust immunostimulatory effect. In addition to influenza vaccines, we have also conducted research related to the development of vaccines against COVID-19 and Ebola virus disease.
Staff
- Prof.: Tokiko Watanabe
- SA Assoc.Prof.: Tomomi Nakahara
- Asst. Prof.: Shintaro Shichinohe
- Asst. Prof.: Itsuki Anzai
- JSPS Postdoc.: Hiroko Kobayashi
Website
Publications
(1) Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Imai M., et al. Proc Natl Acad Sci USA. 2020, 117(28):16587-16595.
(2) Villains or heroes? The raison d’tre of viruses. Watanabe T, Kawaoka Y. Clin Transl Immunology. 2020, 9(2):e01114.
(3) A Highly Pathogenic Avian H7N9 Influenza Virus Isolated from A Human Is Lethal in Some Ferrets Infected via Respiratory Droplets. Imai M, Watanabe T, Kiso M et al. Cell Host Microbe. 2017, 22(5):615-626.e8.
(4) Influenza virus-host interactome screen as a platform for antiviral drug development. Watanabe T., et al. Cell Host Microbe. 16: 795-805. 2014.
(5) Circulating avian influenza viruses closely related to the 1918 virus have pandemic potential. Watanabe T, Zhong G, Russell CA et al. Cell Host Microbe. 15: 692-705. 2014.
(6) Characterization of H7N9 influenza A viruses isolated from humans. Watanabe T., et al. Nature. 501:551-5. 2013.
- Home
- Laboratories
- Watanabe Lab