Ebina Lab/BIKEN Innovative Vaccine Research Alliance Laboratories Virus Vaccine Group
It is difficult to develop vaccines against pathogens that cannot be suppressed by antibody-mediated immunity alone, pathogens that cannot be cultured, or those whose infection is difficult to evaluate due to the absence of animal models. The Viral Vaccine Project is conducting virus research with the aim of developing here in Japan, the world's first vaccines targeting these infectious diseases.
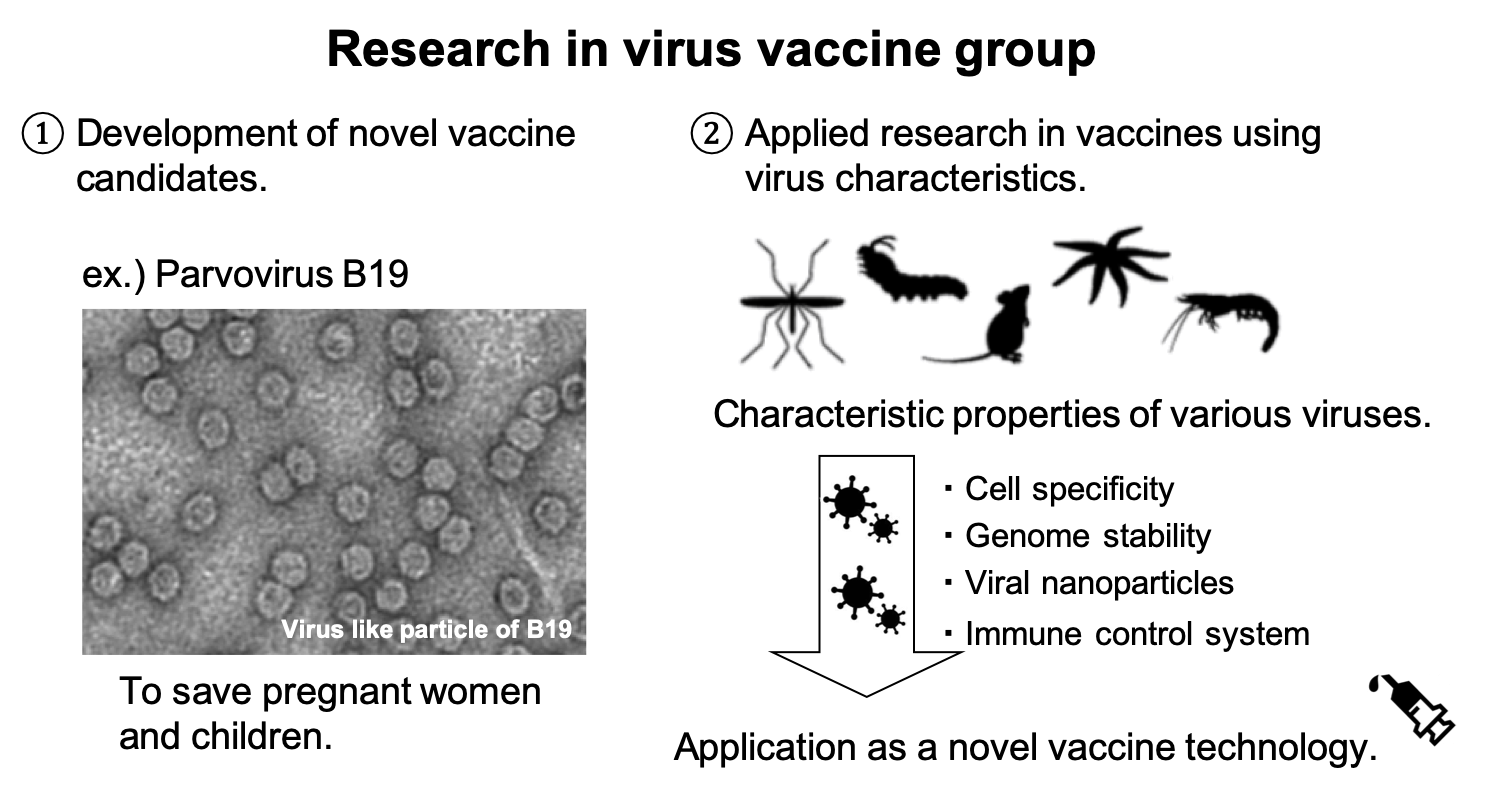
Human parvovirus B 19 infection in pregnant women causes Hydrops fetalis due to severe fetal anemia. Therefore, vaccines are needed but have not yet been developed. However, no virus culture method has been established, the virus host is limited to humans, and there is no adequate animal model available. We are developing novel parvovirus vaccines using various approaches, such as analyzing parvovirus replication mechanism and performing human epidemiological research.
Viruses that replicate in a variety of organisms, apart from humans, have unique properties that can be applied as vaccines for humans. We are conducting virus research with the aim of creating powerful, safe, and novel vaccine platforms by taking advantage of the characteristics of various viruses.
Staff
- SA Prof.: Hirotaka Ebina(concur.)
Publications
- (1) Immunogenicity and safety of a live-attenuated SARS-CoV-2 vaccine candidate based on multiple attenuation mechanisms. Suzuki-Okutani M., et al., bioRxiv doi: https://doi.org/10.1101/2024.03.03.582873
(2) Protocol to isolate temperature-sensitive SARS-CoV-2 mutants and identify associated mutations. Okamura S., et al., STAR Protoc. (2023) May 15;4(2):102352.
(3) Versatile live-attenuated SARS-CoV-2 vaccine platform applicable to variants induces protective immunity. Yoshida A., et al., iScience (2022) Nov 18;25(11):105412.
(4) Safety and immunogenicity of parvovirus B19 virus-like particle vaccine lacking phospholipase A2 activity. Suzuki H., et al., Vaccine (2022) 40(42):6100-6106
(5) Could live attenuated vaccines better control COVID-19? Okamura S., et al., Vaccine (2021) Sep 15;39(39):5719-5726.v
(6) Fusion of parvovirus B19 receptor-binding domain and pneumococcal surface protein A induces protective immunity against parvovirus B19 and Streptococcus pneumoniae. Suzuki H., et al., Vaccine (2021) 39(36):5146-5152
- Home
- Laboratories
- Ebina Lab