Horii Lab/Research Center for Infectious Disease Control Laboratory of Malaria Vaccine Development
Malaria is widespread in tropical and subtropical regions, and millions of people, particularly in Africa, remain at risk of disease and death despite substantial progress in malaria control. Our laboratory is undertaking basic research and conducting clinical trials on our own candidate vaccine antigen gene.
Malaria vaccine targeting SERA
The treatment of malaria patients is completely dependent on the efficacy of anti-malaria drugs, however, drug-resistant parasites are emerging. Although a malaria vaccine is the ideal weapon against this pathogen, vaccine development is hampered by genetic polymorphisms in candidate antigen genes.
We have been focusing on P. falciparum SERA5 molecule whose gene is conserved worldwide and developing malaria vaccine NPC-SE36 by utilizing a recombinant SE36 protein. Epidemiological studies in malaria hyper-endemic areas showed that children with antibodies against SE36 experienced few or no symptomatic/clinical malaria, albeit such children are a minority.
It was surprising that Ugandan adults that suffered numerous malaria infections did not respond to vaccination with NPC-SE36. By contrast, malaria-naive Japanese adults produced high levels of antibodies. Moreover, we observed good antibody response in young Ugandan children that experienced few malaria episodes. We obtained 72 % protective efficacy 1 year post-2nd-vaccination in a follow-up study of 6-20 years old in the phase lb trial. We have conducted Phase lb clinical trial of NPC-SE36 in Burkina Faso in west Africa in 2015-2017. Vaccine was well tolerated, and it was found that the immune response in 1 year infant group was much higher than children 2-5 years old. We have also completed Phase lb clinical trial (adult to 1 year baby) of NPC-SE36 with CpG adjuvant that stimulates innate immunity resulting in a significant immune response without safety concern and 74% protective efficacy. Phase II clinical trial is under planning.
Molecular strategy for malaria parasite survival and a function of SE36 protein
The malaria parasite develops highly sophisticated strategies to evade the human immune system. One of the most difficult phenomena encountered by those developing vaccines is genetic polymorphism of vaccine candidate genes. Fortunately, SE36 gene sequence is highly conserved among malaria parasites worldwide. Recently we have shown that SE36 protein tightly binds to host vitronectin as cytoadherence molecule on the surface of parasite cell, merozoite, and vitronectin further binds to over 30 different host proteins for molecular camouflage from host immune system. Presentation of SE36/vitronectin complex to host immune system by repeated infections may cause immune tolerance against SE36 protein. Thus, limited genetic polymorphism of SE36 is presumably resulted from lower immune response.
About Malaria Vaccine Development
-
Fig. 1. Clinical trial of the NPC-SE36 malaria vaccine. The vaccine was produced under GMP (Good Manufacturing Practice) conditions at the Kanonji Institute of The Research Foundation for Microbial Diseases of Osaka University. (NPC-SE36 malaria vaccine was previously called BK-SE36 malaria vaccine.)
-
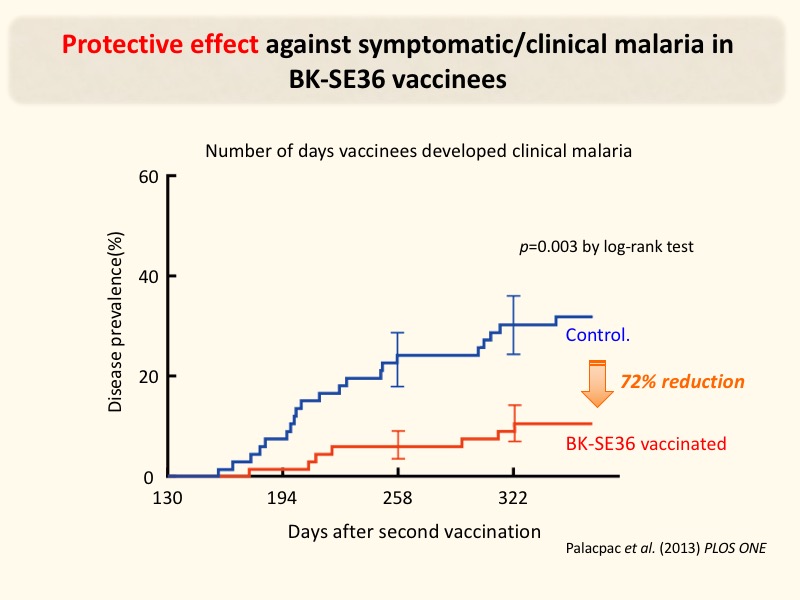
Fig. 2. Protective efficacy of NPC-SE36 malaria vaccine
A one-year follow-up study was conducted on the vaccine group (66 subjects) who received the NPC-SE36 malaria vaccine and the control group (66 subjects). The cumulative number of malaria cases in the vaccine was lower than in the control group with 72% protective efficacy. Palacpac et al., Plos ONE. 2013; 8(5): e64073
-
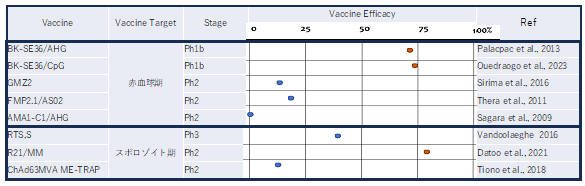
Fig. 3. Comparison of the protective efficacy of major malaria vaccine candidates reported so far around the world
The protective efficacies of erythrocytic stage vaccines other than SE36 are low because the antigen genes used are highly polymorphic. The sporozoite stage vaccines RTS,S and R21 have been pre-qualified by WHO. However, unlike the SE36 vaccine, an annual booster vaccination is required.
Staff
- Head, Prof. : Tetsuya Iida(concur.)
- SA.Prof.: Toshihiro Horii
- SA.Prof.: PALACPAC NIRIANNE MARIE QUERIJERO
Website
Publications
(1)Persistence of Anti-SE36 Antibodies Induced by the Malaria Vaccine Candidate BK-SE36/CpG in 5-10-Year-Old Burkinabe Children Naturally Exposed to Malaria. Nebie I, et al., Vaccines (Basel). (2024) 12(2):166.
(2)Safety and immunogenicity of BK-SE36/CpG malaria vaccine in healthy Burkinabe adults and children: a phase 1b randomised, controlled, double-blinded, age de-escalation trial. Ouédraogo A, et al., Front Immunol. (2023) 14:1267372.
(3)Plasmodium falciparum infection coinciding with the malaria vaccine candidate BK-SE36 administration interferes with the immune responses in Burkinabe children. Tiono AB, et al., Front Immunol. (2023) 14:1119820.
(4)African-specific polymorphisms in Plasmodium falciparum serine repeat antigen 5 in Uganda and Burkina Faso clinical samples do not interfere with antibody response to BK-SE36 vaccination. Arisue N et al., Front Cell Infect Microbiol. (2022) 12:1058081.
(5)Safety and immunogenicity of BK-SE36 in a blinded, randomized, controlled, age de-escalating phase Ib clinical trial in Burkinabe children. Bougouma EC, et al., Front Immunol. (2022) 13:978591.
(6)First-in-human randomized trial and follow-up study of Plasmodium falciparum blood-stage malaria vaccine BK-SE36 with CpG-ODN(K3). Ezoe S, et al., Vaccine. (2020)
(7)The malaria parasite Plasmodium falciparum in red blood cells selectively takes up serum proteins that affect host pathogenicity. Tougan T, et al., Malar J. (2020) 19(1):155.
(8)Molecular Camouflage of Plasmodium falciparum Merozoites by Binding of Host Vitronectin to P47 Fragment of SERA5. Tougan T, et al., Sci Rep. (2018) 8(1):5052.
(9)Antibody titres and boosting after natural malaria infection in BK-SE36 vaccine responders during a follow-up study in Uganda. Yagi M, et al., Sci Rep. (2016) 6:34363.
(10)Protective Epitopes of the Plasmodium falciparum SERA5 Malaria Vaccine Reside in Intrinsically Unstructured N-Terminal Repetitive Sequences. Yagi M, et al., PLoS One.(2014) 9(6):e98460.
- Home
- Laboratories
- Horii Lab