Crystal structure of Ragulator determined (Okada Lab, in Nat.Commun)
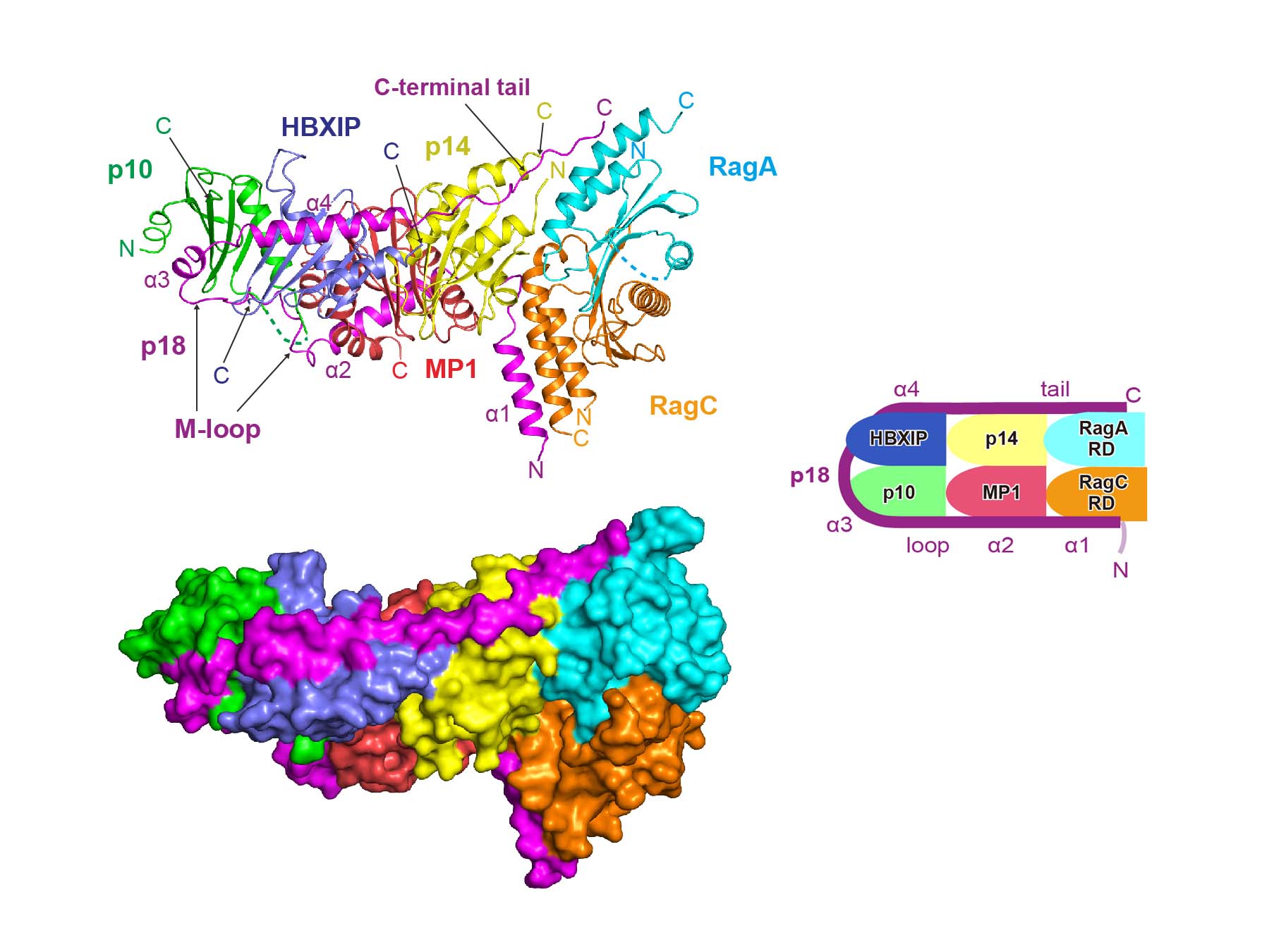
The mechanistic target of rapamycin complex 1 (mTORC1) plays a central role in regulating cell growth and metabolism by responding to cellular nutrient conditions. The activity of mTORC1 is controlled by Rag GTPases, which are anchored to lysosomes via Ragulator, a pentameric protein complex consisting of membrane-anchored p18/LAMTOR1 and two roadblock heterodimers. To elucidate the molecular basis for the regulation of Rag GTPases, we determined the crystal structure of Ragulator in complex with the roadblock domains of RagA-C. In the structure, p18 adopts a novel fold by induced fit, wrapping around the three pairs of roadblock heterodimers to tandemly assemble them onto lysosomes. Cellular and in vitro analyses further demonstrated that p18 is required for Ragulator-Rag GTPase assembly and amino acid-dependent activation of mTORC1. These results establish p18 as a critical organising scaffold for the Ragulator-Rag GTPase complex, which may provide a platform for nutrient sensing on lysosomes.
This research project was conducted in collaboration with Nakagawa Lab. IPR Osaka University., Nakayama Lab. Medical Institute of Bioregulation, Kyushu University, and Daron Lab. RIMD Osaka University.
This article was published in Nature Communications in Nov 20, 2017
Structural basis for the assembly of the Ragulator-Rag GTPase complex.
Naturre Communications website
- Home
- Achievement
- Research Activities
- Crystal structure of Ragulator determined (Okada Lab, in Nat.Commun)