Essential role for GABARAP autophagy proteins in interferon-inducible GTPase-mediated host defense (Yamamoto Lab, in Nat Immunolgy)
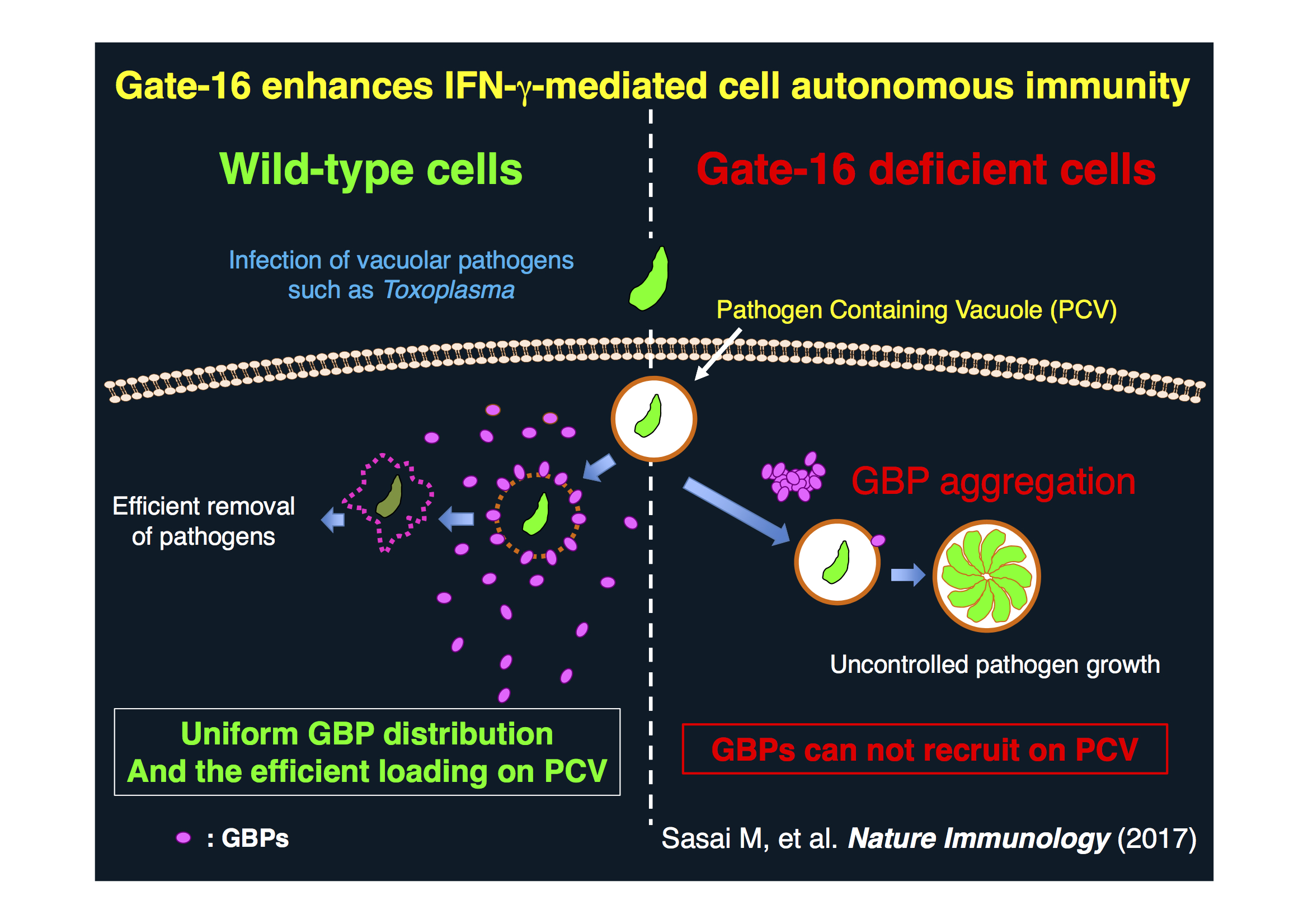
Although functions of autophagy-related (Atg) proteins in canonical autophagy are well understood, recent studies have revealed roles of Atg proteins in various other biological processes. While many of Atg genes are highly conserved throughout evolution, the Atg8 gene has undergone expansion in the genome of higher eukaryotes. Mammalian Atg8 homologues consist of LC3s and GABARAPs, all of which are well known to be involved in canonical autophagy. In contrast, the roles of Atg8 homologue member in non-canonical processes are not fully understood. Here we show a unique role of GABARAPs, in particular Gate-16, in interferon-γ (IFN-γ)-mediated antimicrobial response. Cells lacking GABARAPs but not LC3s are defective in IFN-γ-induced clearance of vacuolar pathogens such as Toxoplasma. Gate-16, but not LC3b, specifically associates with Arf1 to mediate uniform distribution of IFN-inducible GTPases. Lack of GABARAPs reduces Arf1 activation, leading to formation of IFN-inducible GTPase-containing aggregates, hampering their function. Furthermore, mice lacking Gate-16 alone, either systemically or specifically in the myeloid compartment, are susceptible to Toxoplasma. Thus, GABARAPs are uniquely required for antimicrobial host defense through cytosolic distribution of IFN-inducible GTPases.
Figs.
(Above) Gate-16 enhances IFN-g-mediated cell autonomous immunity
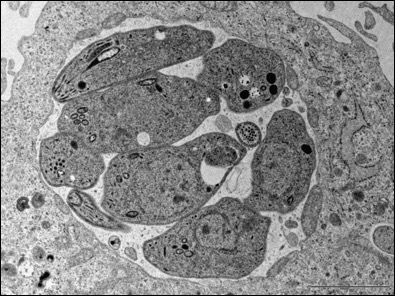
(Below) EM micrograph for Toxisoplasma gondi in Pathogen Containing Bacuole
Original Article
Essential role for GABARAP autophagy proteins in interferon-inducible GTPase-mediated host defense
Nature Immunology 2016, doi: 10.1038/ni.3767
Links
- Home
- Achievement
- Research Activities
- Essential role for GABARAP autophagy proteins in interferon-inducible GTPase-mediated host defense (Yamamoto Lab, in Nat Immunolgy)