New method to study embryonic implantation offers hope for assisted reproduction (Ikawa Lab, in Nat. Commun.)
The use of assisted reproductive technologies like in vitro fertilization is becoming more common worldwide. However, while these technologies successfully create viable embryos, a little over half of all embryos are lost because they fail to implant into the uterus.
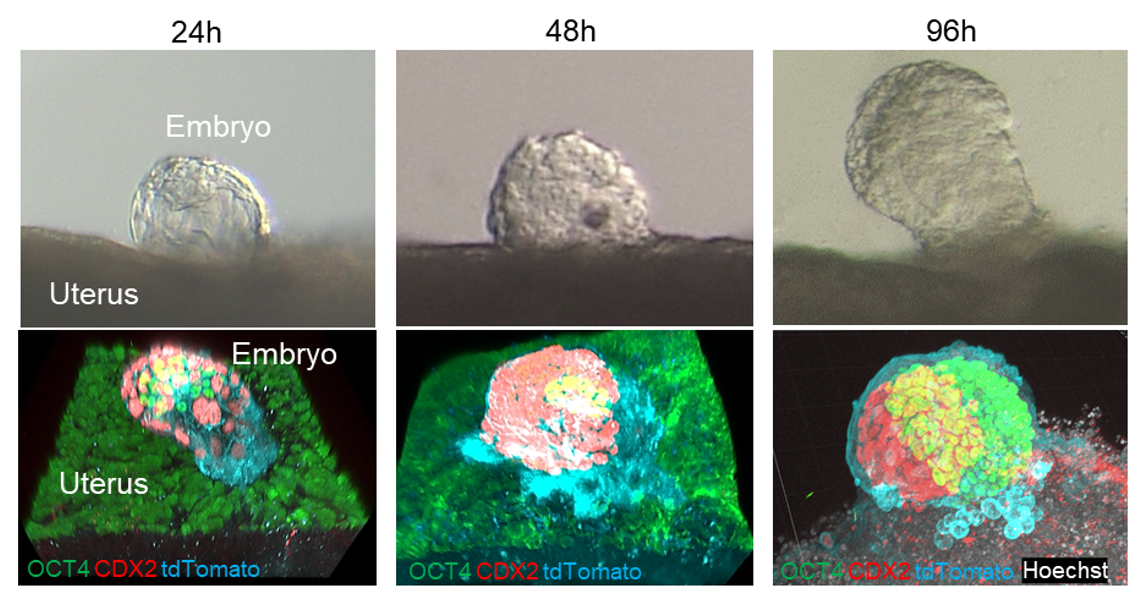
Now, in an article published recently in Nature Communications, researchers describe a technique for studying embryonic implantation in mice by using uterine tissue outside the body (or “ex vivo”), which they hope will lead to improved implantation rates in humans.
Studying implantation is inherently more difficult than the prior stages of in vitro fertilization because it requires observing deep tissues in a live uterine environment. These challenges made researchers wonder: what if they could find a way to keep part of the uterus alive outside of the body, so that implantation could be observed without any barriers?
“Previous studies have used model embryos, created from stem cells, to emulate embryonic development pre- and post-implantation,” explains lead author, Takehiro Hiraoka. “However, without the uterus, they cannot genuinely replicate embryo implantation, and studies have been unable to recreate this process.”
Using a specialized culture method, in which mouse uterine tissue is positioned between liquid and gas surfaces on either side, the researchers were able to place mouse embryos onto small pieces of endometrium tissue. They could then evaluate how the embryos implanted and developed. Impressively, their technique had over 90% efficacy for implantation, which was followed by embryo development and invasion of the uterine lining by the embryo.
“We also saw some features that are characteristic of what happens in implantation inside the body,” says Masahito Ikawa, senior author. “For example, the maternal implantation regulator COX-2 was induced at the site of embryonic attachment.”
To further highlight the potential of their system, the research team looked at the downstream pathways of COX-2 induction. They found that embryonic AKT, a protein that is involved in placental formation and arrangement as well as in cell survival, migration, and invasion, was affected by maternal COX-2.
“Further experiments indicated that introducing an activated form of AKT into embryos recovered defective implantation that was triggered by maternal COX-2 inhibition,” confirms Ikawa. “We were thus able to find a potential way to overcome the issue of implantation failure, indicating the strong potential of our technique for improving assisted reproduction in the future.”
Although challenges remain, such as how to keep the embryos developing past embryonic day 5.5, the results are promising. With future methodological improvements, this technique will allow for the development of treatments for people with recurrent implantation failure. It may also improve implantation rates in assisted reproductive technologies, thereby allowing families with previously untreatable conditions to achieve their dreams.
This article was published in Nature Communications on July 1, 2025
Title: “An ex vivo uterine system captures implantation, embryogenesis, and trophoblast invasion via maternal-embryonic signaling”
Authors: Takehiro Hiraoka, Shizu Aikawa, Daisuke Mashiko, Tatsuya Nakagawa, Hiroki Shirai, Yasushi Hirota, Hiroshi Kimura, Masahito Ikawa
Links
- Home
- Achievement
- Research Activities
- New method to study embryonic implantation offers hope for assisted reproduction (Ikawa Lab, in Nat. Commun.)