Extracellular amino acid influx via LAT1 regulates cellular sensitivity to a transferrin-receptor 1 inhibitory antibody (PPMX-T003) of aggressive natural killer cell leukemia (Kotani Lab, in Leukemia)
Aggressive natural killer cell leukemia (ANKL) is a rare hematological malignancy associated with reactivation of Eptein-Barr virus and shows fulminant clinical course with the overall survival of less than two months. Although several retrospective analyses indicate the effectiveness of L-asparaginase-containing intensive chemotherapy followed by allogeneic hematopoietic cell transplantation for improvement of the overall survival period, poor general appearance with severe liver injury due to disease makes it difficult to apply enough dosage of cytotoxic agents including L-asparaginase and results in poor outcomes.
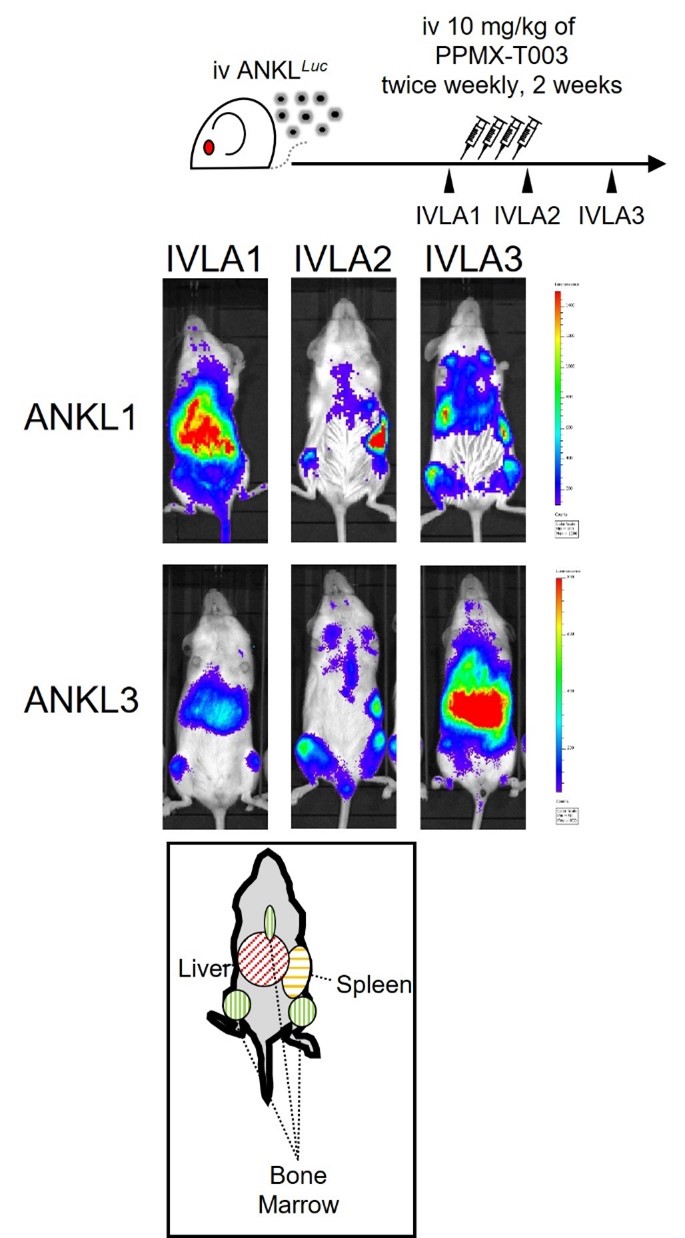
We previously reported that ANKL proliferates mainly in the liver sinusoid depending on supplementation of transferrin, an iron carrier protein, from hepatocytes, and an anti-transferrin receptor 1 (TfR1) inhibitory antibody PPMX-T003 have an antineoplastic potential against the liver-resident ANKL cells (Kameda, Yanagiya, Miyatake et al. Blood. 2023). However, ANKL cells resident in extrahepatic organs, especially the spleen, resist to PPMX-T003, indicating that cellular sensitivity to PPMX-T003 of ANKL cells depends on the microenvironmental factors of which they resident in. In this study, we aimed to uncover the factor (Figure 1).
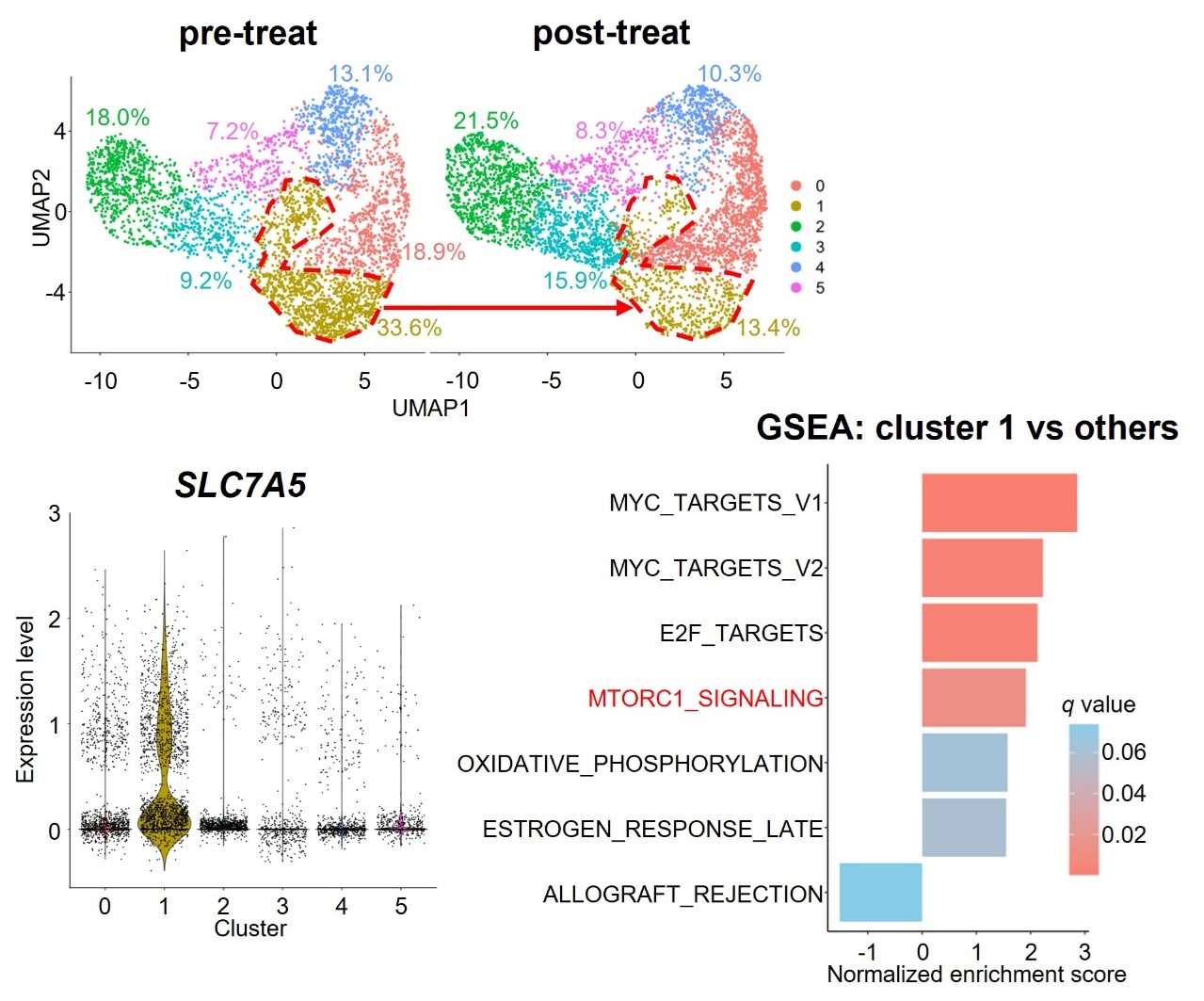
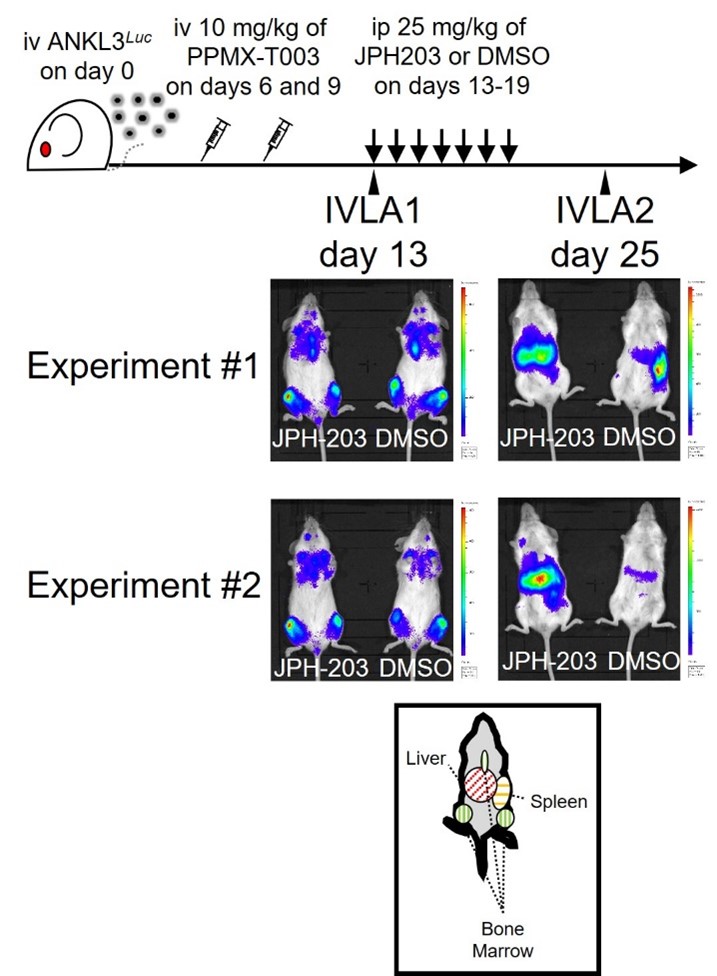
The single cell RNA sequencing analysis of the ANKL cells derived from pre- and post-treated patient-derived xenograft mouse models with PPMX-T003 demonstrated that the PPMX-T003 sensitivity cluster consists of ANKL cells characterized by specifically higher expression of LAT1 (SLC7A5), a large neutral amino acid transporter, accompanied with mTORC1 signal activation (Figure 2). Furthermore, LAT1 inhibition with JPH-203 decrease the antineoplastic effect of PPMX-T003 against ANKL cells (Figure 3), suggesting that extracellular amino acid influx via LAT1 regulates the cellular sensitivity to PPMX-T003 of ANKL. Since the nutrients absorbed from gut pass the sinusoidal vessels to reach the hepatocytes, ANKL cells resident in the liver expose to higher concentration of amino acids than those in the spleen and show higher sensitivity to PPMX-T003.
This work was published from the journal Leukemia on 24 June, 2024.
Title: Amino acid influx via LAT1 regulates iron demand and sensitivity to PPMX-T003 of aggressive natural killer cell leukemia
Authors: Ryo Yanagiya, Yuji Miyatake, Natsumi Watanabe, Takanobu Shimizu, Akane Kanamori, Masaya Ueno, Sachiko Okabe, Joaquim Carreras, Shunya Nakayama, Ami Hasegawa, Kazuaki Kameda, Takeshi Kamakura, So Nakagawa, Takuji Yamauchi, Takahiro Maeda, Keisuke Ishii, Tadashi Matsuura, Hiroshi Handa, Atsushi Hirao, Kenichi Ishizawa, Makoto Onizuka, Tetsuo Mashima, Naoya Nakamura, Kiyoshi Ando, Ai Kotani* (*Corresponding author)
DOI: 10.1038/s41375-024-02296-6
Links
-
Figure 1. In vivo luciferase assays (IVLAs) of luciferase-overexpressed two lineages of ANKL patient-derived xenograft mice (ANKL1 and ANKL3). After treatment with PPMX-T003, ANKL cells remnant in the extrahepatic organs, including the spleen and the bone marrow.
-
Figure 2. The single cell RNA sequencing analysis of ANKL cells derived from pre- and post-treated patient-derived xenograft mice with PPMX-T003. The PPMX-T003 sensitive cluster (Cluster 1) consists of the ANKL cells with higher expression of LAT1 (SLC7A5) and activation of mTORC1 signaling pathway.
-
Figure 3. In vivo luciferase assays of the patient-derived xenograft mice treated with PPMX-T003 and JPH-203 or DMSO. At IVLA2 stage, JPH-203 exposed mice show stronger luciferin luminescence derived from the liver, indicating the decrease of antineoplastic effect of PPMX-T003 due to LAT1 inhibition.
- Home
- Achievement
- Research Activities
- Extracellular amino acid influx via LAT1 regulates cellular sensitivity to a transferrin-receptor 1 inhibitory antibody (PPMX-T003) of aggressive natural killer cell leukemia (Kotani Lab, in Leukemia)