Overcoming Senolytic Drug Resistance in Senescent Cells through Mitochondrial Control (Hara Lab, in Nature Aging)
Summary
A research team led by Professor Eiji Hara and Assistant Professor Masahiro Wakita (RIMD, IFReC and CiDER, The University of Osaka) has systematically compared the efficacy of 21 senolytic drugs and identified a subpopulation of senolytic-resistant cells. The team demonstrates a mitochondrial basis for senolytic resistance and shows that metabolic interventions markedly enhance senescent cell clearance in vivo.
Research Background and Results
Cellular senescence is a stable form of cell-cycle arrest triggered by a variety of potentially oncogenic stresses, such as telomere erosion, oxidative stress, radiation, or oncogene activation. While this response prevents the expansion of cells at risk of malignant transformation and thus acts as a tumor-suppressive mechanism, senescent cells also have a darker side. They secrete pro-inflammatory factors collectively known as the senescence-associated secretory phenotype (SASP), which can promote tissue dysfunction and disease depending on the biological context. Accordingly, the targeted elimination of senescent cells by “senolytic” drugs has emerged as a promising therapeutic strategy.
Over the past decade, more than 20 candidate senolytic drugs have been reported, spanning diverse mechanisms of action. However, despite this growing list, no systematic head-to-head comparison of their efficacies and specificities has been performed. As a result, it has remained unclear which agents most effectively eliminate senescent cells while sparing non-senescent counterparts. Furthermore, even the most potent senolytic drugs fail to eliminate a subset of resistant cells, but the mechanisms underlying this resistance have been poorly understood.
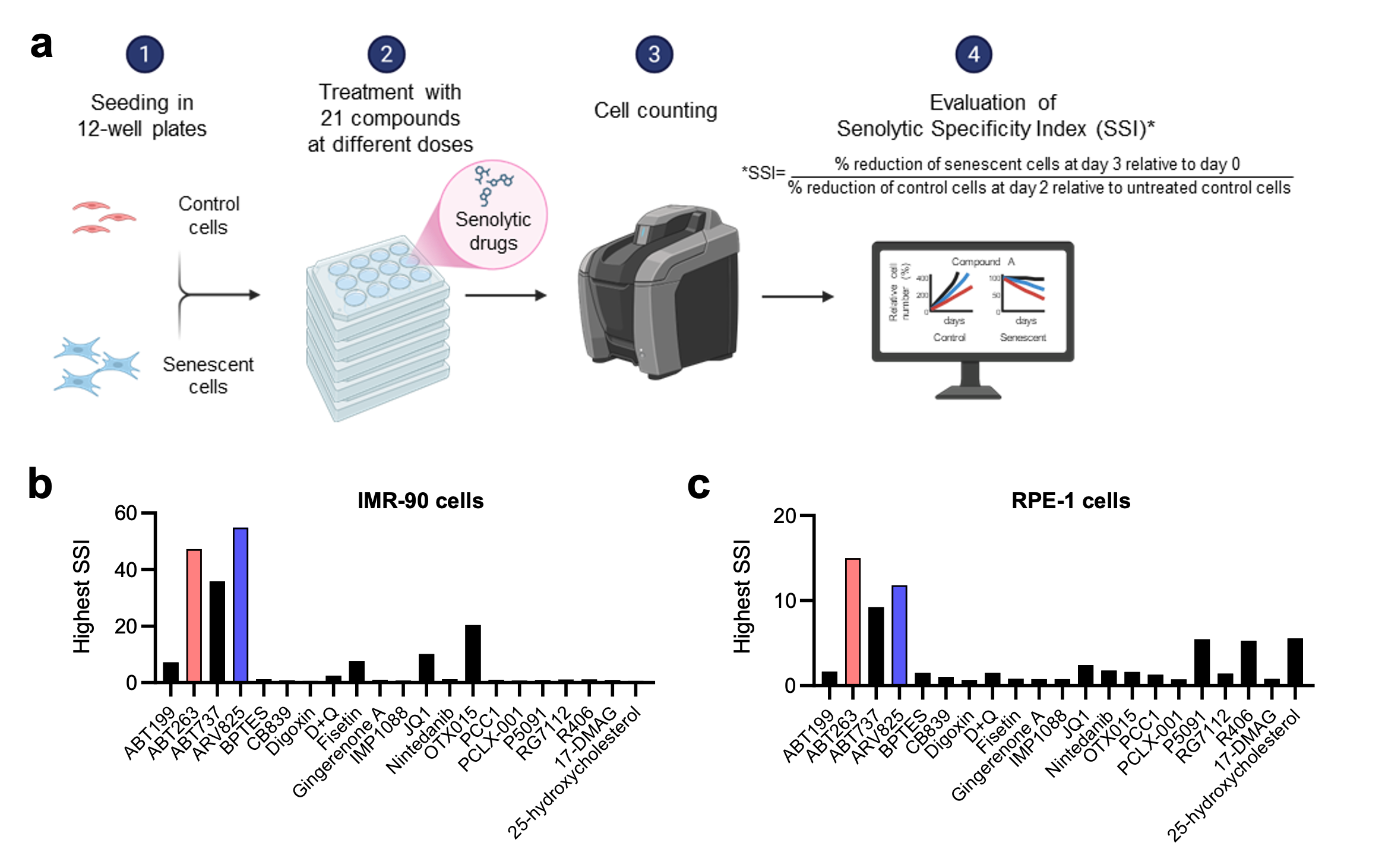
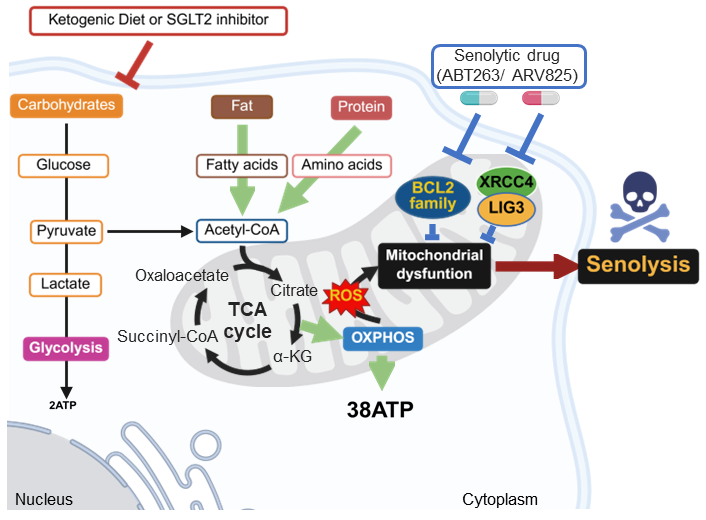
In this study, Wakita et al. systematically compared 21 senolytic agents using a quantitative Senolytic Specificity Index (SSI). This analysis identified the Bcl-2 inhibitor ABT263 and the BET inhibitor ARV825 as the most effective senolytics across fibroblast and epithelial models (Fig. 1). However, even upon extended treatment with these most potent senolytics, a proportion of senescent cells remained viable. Wakita et al found that senolytic resistance was driven by maintenance of mitochondrial integrity through V-ATPase–mediated clearance of damaged mitochondria. Imposing mitochondrial stress via metabolic shit from glycolysis to OXPHOS enhanced the senolytic efficacies of ABT263 and ARV825 in vitro and in mouse models, ketogenic diet adoption or SGLT2 inhibition similarly potentiated ABT263- and ARV825-induced senolysis, reducing tumour growth and metastasis. These findings suggest that mitochondrial quality control is a key determinant of resistance to ABT263- and ARV825-induced senolysis, providing a possible framework for rational combination senotherapies (Fig. 2).
*This study was published online in Nature Aging on January 29, 2026.
Title: Comparative analysis of senolytic drugs reveals mitochondrial determinants of efficacy and resistance.
Authors: Masahiro Wakita, Koyu Ito, Kaho Fujii, Dai Sakamoto, Takumi Mikawa, Sho Sugawara, Xiangyu Zhou, Jeong Hoon Park, Hideka Miyagawa, Daisuke Motooka, Emi Ogasawara, Naotada Ishihara, Akiko Takahashi, Hiroshi Kondoh, and Eiji Hara
Links
-
Fig. 1 | Comparative analysis of senolytic drugs revealed ABT263 and ARV825 as the most potent compounds.
a, Outline of the comparative analysis of senolytic drug. Early passage HDFs (IMR-90 cells) and RPE-1 epithelial cells were rendered senescent by serial passaging (Rep-Sen cells) or treatment with 150 ng/ml doxorubicin for 10 days (DXR-Sen cells), respectively. Senescent and non-senescent (control) cells were treated with 21 compounds at multiple concentrations for 3 days. Relative cell numbers were determined throughout the experiments. The Senolytic Specificity Index (SSI) was calculated as: SSI = (% reduction of senescent cells at day 3 relative to day 0) / (% reduction of control cells at day 2 relative to untreated control cells). If the reduction of control cells at day 2 was <1%, the denominator was set to 1. Created with BioRender.com. b and c, For each compound, the SSI value at the concentration yielding the highest index is shown.
-
Fig. 2 | Model for enhancing the senolytic activity of ABT263 and ARV825 by metabolic shift.
Ketogenic diets and SGLT2 inhibitors lower blood glucose levels, thereby suppressing glycolysis with compensatory upregulation of OXPHOS. This increases the mitochondrial workload and induces mitochondrial stress, thereby enhancing the vulnerability of mitochondria to mitochondria-targeting senolytic drugs such as ABT263 and ARV825.
- Home
- Achievement
- Research Activities
- Overcoming Senolytic Drug Resistance in Senescent Cells through Mitochondrial Control (Hara Lab, in Nature Aging)