Transferrin and ferritin regulate cell cycle of lymphoma cells at different points (Kotani Lab, Redox Biology)
Although is widely known that iron is important for the survival and proliferation of cells in the human body, especially tumor cancer cells, there has been no comprehensive analysis of the contribution of iron in the cell cycle regulation. In particular, there are two types of iron existences that are mainly used by cells: transferrin (extracellular iron supplements), and ferritin (intracellular iron storage). The functional differences between these two types of iron have not been investigated in detail except in terms of transport and storage.
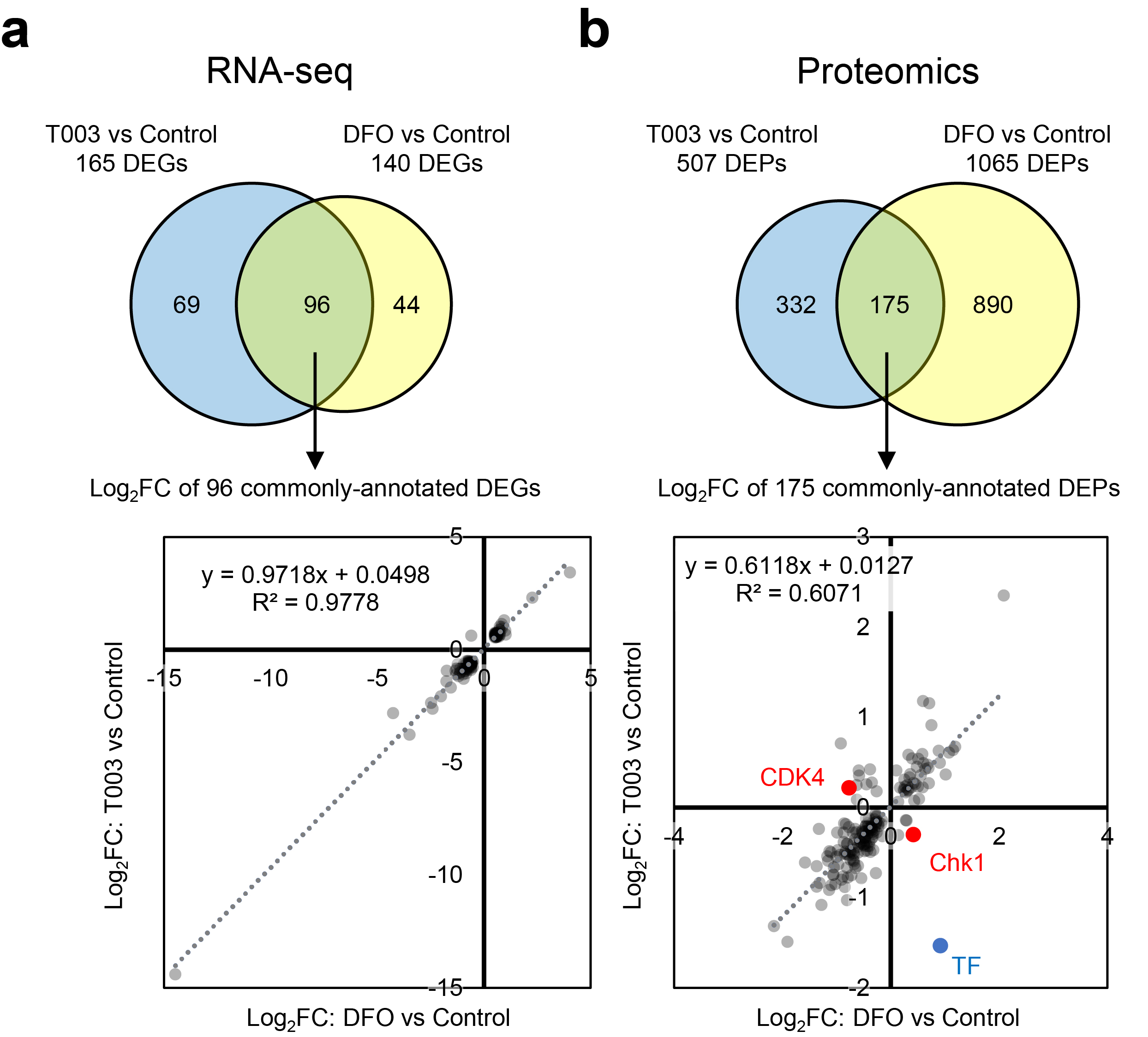
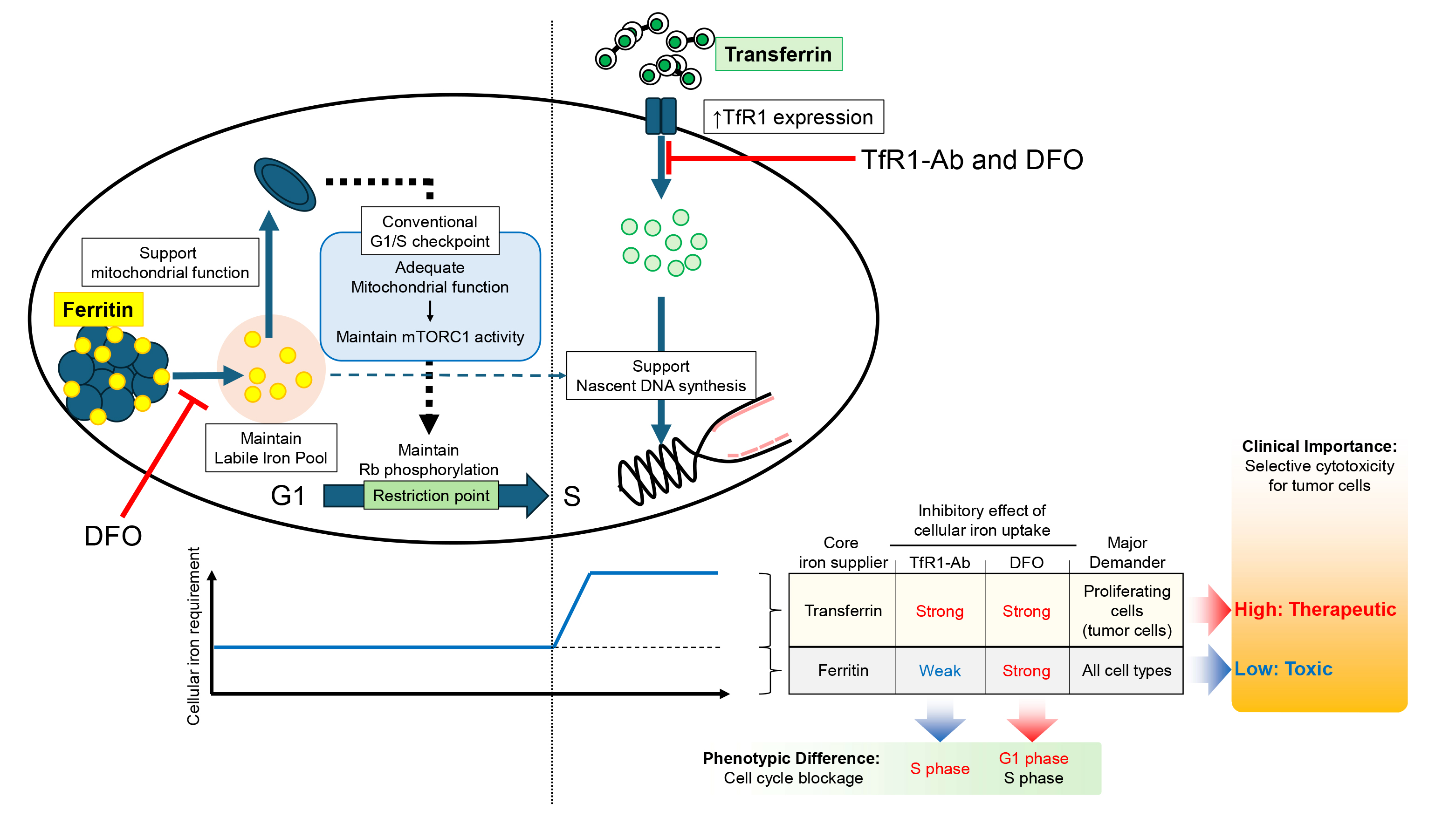
Prof. Kotani and her lab members previously demonstrated that PPMX-T003, a transferrin receptor inhibitory antibody, has remarkable antineoplastic activity against aggressive NK cell leukemia, a fulminant subtype of mature NK cell lymphoma, and explored its underlying molecular mechanisms. We found that cells selectively depleted of only transferrin-derived iron by PPMX-T003 arrest the cell cycle at a different site than cells depleted of both transferrin- and ferritin-derived iron by conventional iron chelating agents. To investigate the underlying mechanisms, we searched for differentially expressed genes whose expression varied between the two by RNA sequencing, but were unable to identify any genes whose expression behavior was clearly different. In cooperation with the Central Instrumentation Laboratory, we comprehensively identified proteins whose expression varied between the two by using a high-depth proteomics, and found that mitochondrial function is specifically impaired in cells depleted of iron stores, resulting in cell cycle arrest in the G1 phase via a decrease in mTORC1 signaling and dephosphorylation of Rb, resulting in cell cycle arrest at the G1 phase. On the other hand, when only transported iron is depleted, the G1 checkpoint are normally overcome, but DNA replication is severely impaired in the S phase of the cell cycle, leading to cell cycle arrest (S phase arrest). These findings indicate that there are functional differences in the roles of transferrin and ferritin in DNA replication and mitochondrial function, respectively.
Mitochondrial function is extremely important not only in cancer cells but also in normal cells because it regulates respiration and energy production. The results of this study suggest that iron chelating agents act on stored iron as well. In contrast, PPMX-T003 is expected to show more selective toxicity against cancer cells because it inhibits the uptake of transported iron by cancer cells while having only a minor effect on iron stores.
The research results were published in the Dutch scientific journal Redox Biology (online) on June 16, 2025.
Title: ”Revised model for cell cycle regulation by iron: differential roles between transferrin and ferritin”
Journal: Redox Biology. 2025; 85: 103727
Authors: Ryo Yanagiya, Hiroko Kato, Akinori Ninomiya, Masaya Ueno, Akane Kanamori, Yuji Miyatake, Masahiro Oka, Keisuke Ishii, Tadashi Matsuura, So Nakagawa, Atsushi Hirao, Makoto Onizuka, and Ai Kotani
Links
-
Figure 1: differentially expressed genes of NK cell lymphoma cells depleted of ferritin (cells after iron chelation with deferoxamine; DFO) and transferrin (cells after transferrin receptor inhibition with PPMX-T003) by RNA sequencing (a), and high-depth proteomics (b). While RNA-seq showed little difference between the two profiles, high-depth proteomics revealed clear differences, mainly in molecules involved in the cell cycle during G1 phase (CDK4) and in molecules related to DNA damage (Chk1).
-
Figure 2: Visual abstract. Ferritin (storage iron: yellow) and transferrin (transport iron: green) regulate the cell cycle in different phases: maintenance of mitochondrial function during G1 phase of the cell cycle and DNA replication during S phase, respectively.
- Home
- Achievement
- Research Activities
- Transferrin and ferritin regulate cell cycle of lymphoma cells at different points (Kotani Lab, Redox Biology)