PLoS Genet. 10:e1004320 2014/05/01
「Discovery of GPI anchor remodeling deficiency」
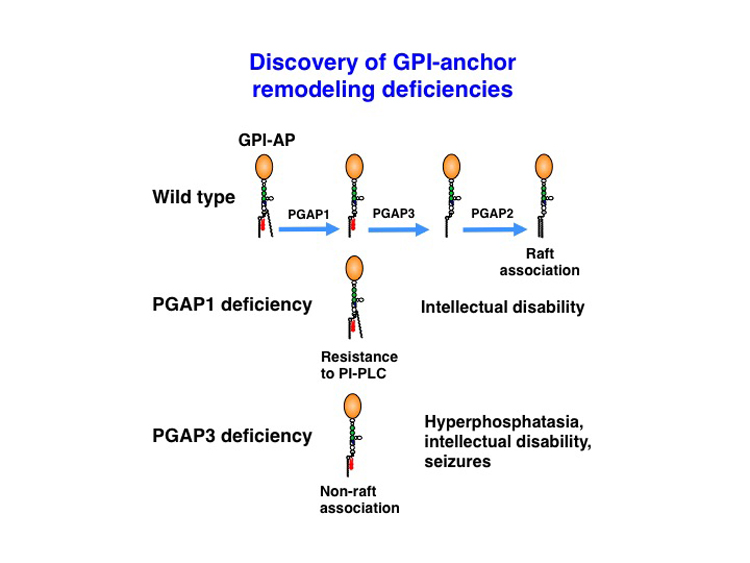
At least 150 different human cell surface proteins are anchored to the plasma membrane via a glycolipid, termed glycosylphosphatidylinositol (GPI). Precursors of GPI-anchored proteins (GPI-AP) generated in the endoplasmic reticulum undergo lipid remodeling mediated by PGAP1, PGAP3 and PGAP2, before becoming mature GPI-AP that are expressed in membrane rafts. In PGAP1 defective cells, inositol-linked fatty acid remains and “three-footed” GPI-AP, which is resistant to bacterial PI-specific phospholipase C, is expressed on the cell surface. In PGAP3 defective cells, GPI-AP with unsaturated fatty acid at the sn2 position, a raft incompatible structure, is expressed on the cell surface. In collaborations with Dr. Jamura and colleagues in Erlangen, we reported that PGAP1 deficiency caused intellectual disability (PLoS Genet., 10(5):e1004320). And in collaboration with Dr. Krawitz and colleagues in Berlin, and Dr. Taylor and colleagues in Oxford, we demonstrated that PGAP3 deficiency caused hyperphosphatasia with intellectual disability and seizures (Am. J. Hum. Genet., 94:278-287). These results show that properly remodeled lipid moiety in GPI-AP is critical for normal development and function of neural system.
Links
- Home
- Achievement
- Research Activities
- PLoS Genet. 10:e1004320 2014/05/01