Nature 495:524-528 2013/03/28
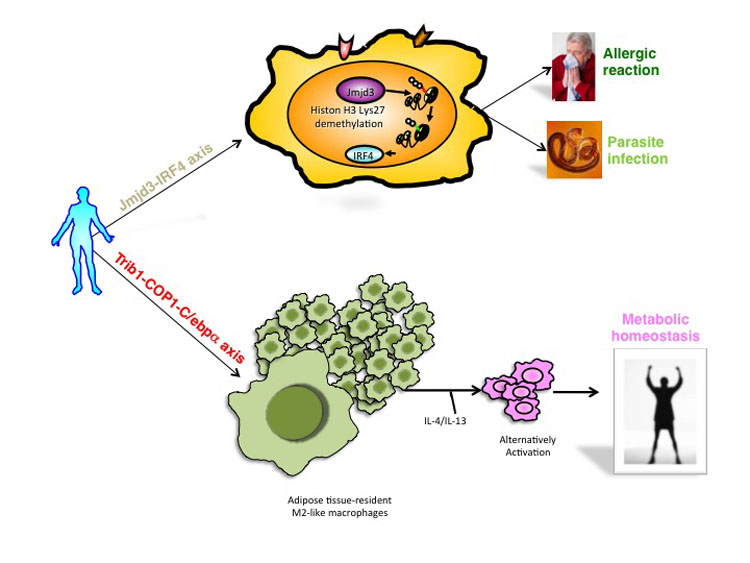
Macrophages are comprised of at least two subgroups, M1 and M2. Whereas M1 macrophages are proinflammatory and play a central role in host defence against bacterial and viral infections, M2 macrophages are associated with responses to helminth infection, tissue remodelling, chronic inflammation, fibrosis and cancer. Trib1 is an adaptor protein involved in protein degradation by interacting with COP1 ubiquitin ligase. Trib1 has been implicated in lipid metabolism by genome-wide association studies (GWAS) in humans. Here, we show that Trib1 is critical for the differentiation of F4/80+MR+ tissue-resident macrophages that share characteristics with M2 macrophages, named M2-like macrophages, and eosinophils but not for M1 myeloid cells. Trib1 deficiency results in a severe reduction of M2-like macrophages in various organs, including bone marrow (BM), spleen, lung and adipose tissues. Aberrant expression of C/EBPα in Trib1-deficient BM cells is responsible for the defects in macrophage differentiation. Unexpectedly, mice lacking Trib1 in haematopoietic cells show lipodystrophy owing to increased lipolysis, even when fed a normal diet. Supplementation of M2-like macrophages rescues the lipodystrophy, indicating a lack of the macrophages is the cause of lipolysis. In response to a high-fat diet, mice lacking Trib1 in haematopoietic cells develop hypertriglyceridaemia, glucose intolerance and insulin resistance, together with increased proinflammatory cytokine gene induction. Collectively, these results demonstrate that Trib1 is critical for adipose tissue maintenance and suppression of metabolic disorders by controlling the differentiation of tissue-resident M2-like macrophages.
- Home
- Achievement
- Research Activities
- Nature 495:524-528 2013/03/28