Cell 139: 352-365 (2009)
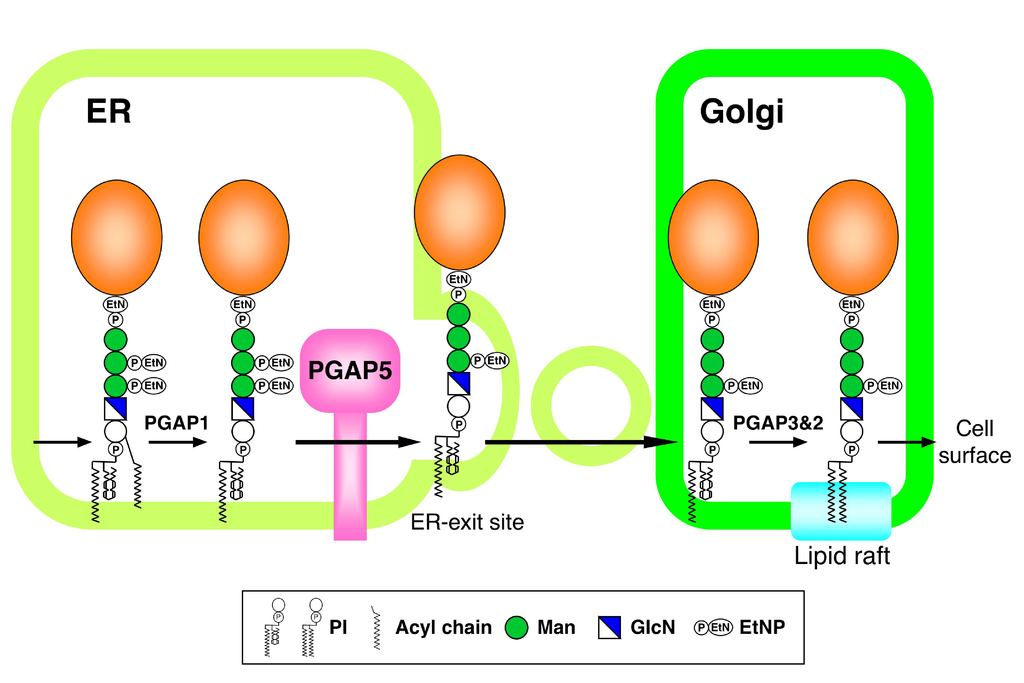
Glycosylphosphatidylinositol (GPI) anchoring of proteins is a conserved post-translational modification in eukaryotes, which is essential for embryonic development, immune responses and neurogenesis in mammals. GPI biosynthesis and attachment to proteins are carried out on the endoplasmic reticulum (ER) membrane. However, how GPI-anchored proteins (GPI-APs) are trafficked from the ER is poorly understood. Here, we established mutant cell lines that were selectively defective in transport of GPI-APs from the ER to the Golgi. We identified a responsible gene, designated PGAP5 (post-GPI-attachment to proteins 5). PGAP5 belongs to a dimetal-containing phosphoesterase family, and catalyzed the remodeling of glycan moiety of GPI-APs: removal of a side-chain ethanolamine-phosphate attached to the second mannose of GPI. PGAP5 appears to function at the ER-exit site, and its catalytic activity is prerequisite for the efficient exit of GPI-APs from the ER. Our data demonstrate that GPI glycan acts as an ER-exit signal and further suggest that glycan remodeling mediated by PGAP5 regulates GPI-AP transport in the early secretory pathway.
Links
- Home
- Achievement
- Research Activities
- Cell 139: 352-365 (2009)