Kamimoto Lab/Division of Cellular and Molecular Biology Department of Systems Biomedical Science
Our bodies consist of various cells and molecules, and their precise regulation controls complex biological functions. Understanding how cells and tissues are formed and maintained is a fundamental question in basic science. Also, the manipulation of genes and cells is essential for applications, such as disease therapy and biomedical engineering.
Our group, the Department of Systems Biomedical Science, aims to unravel the circuits of cells and genes from large-scale and complex data. We integrate omics and single-cell measurement data to construct computational models that recapitulate the biological dynamics of interest in a computer (in silico). Through these models, we aim to decipher the rules of complex biological regulation and use them as predictive simulation models. Through this approach, which we call “in silico life science research,” we will gain insight into fundamental biology, medicine, and bioengineering.
Our research topics include cancer, aging, immunology, and infectious diseases.
In silico reconstruction of the genetic program of disease and development and its use as a predictive model
In recent years, newly developed measurement techniques have made it possible to acquire vast quantities of biological and medical data; however, it is not easy to understand the mechanisms behind such complex and enormous data and to exploit them for medical treatment. For example, the genome sequence, which is the blueprint of our body, has been completely sequenced. However, even though we can sequence and measure the genome DNA, we have not yet elucidated the meaning of these sequences. A “translator” is needed to decipher and utilize the grammar of life that underlies the complex and voluminous measurement data. Computational assistance is also required to understand complex networks and systems, which consist of many interactions between genes and molecules.
We construct models that reproduce the behavior of life on a computer (in silico) by integrating biological data. The technology to simulate and predict complex disease processes is essential to achieve predictive medicine, which may prevent diseases before they occur, and personalized medicine, which selects treatments based on individual differences. Computational simulations can also be used for large-scale screening (exploratory research) to design new molecules and cells beyond the constraints of real-world technologies. Our research group aims to contribute to disease and basic life science research from these informatics perspectives.
Development of new research technology by integrating single-cell omics and AI
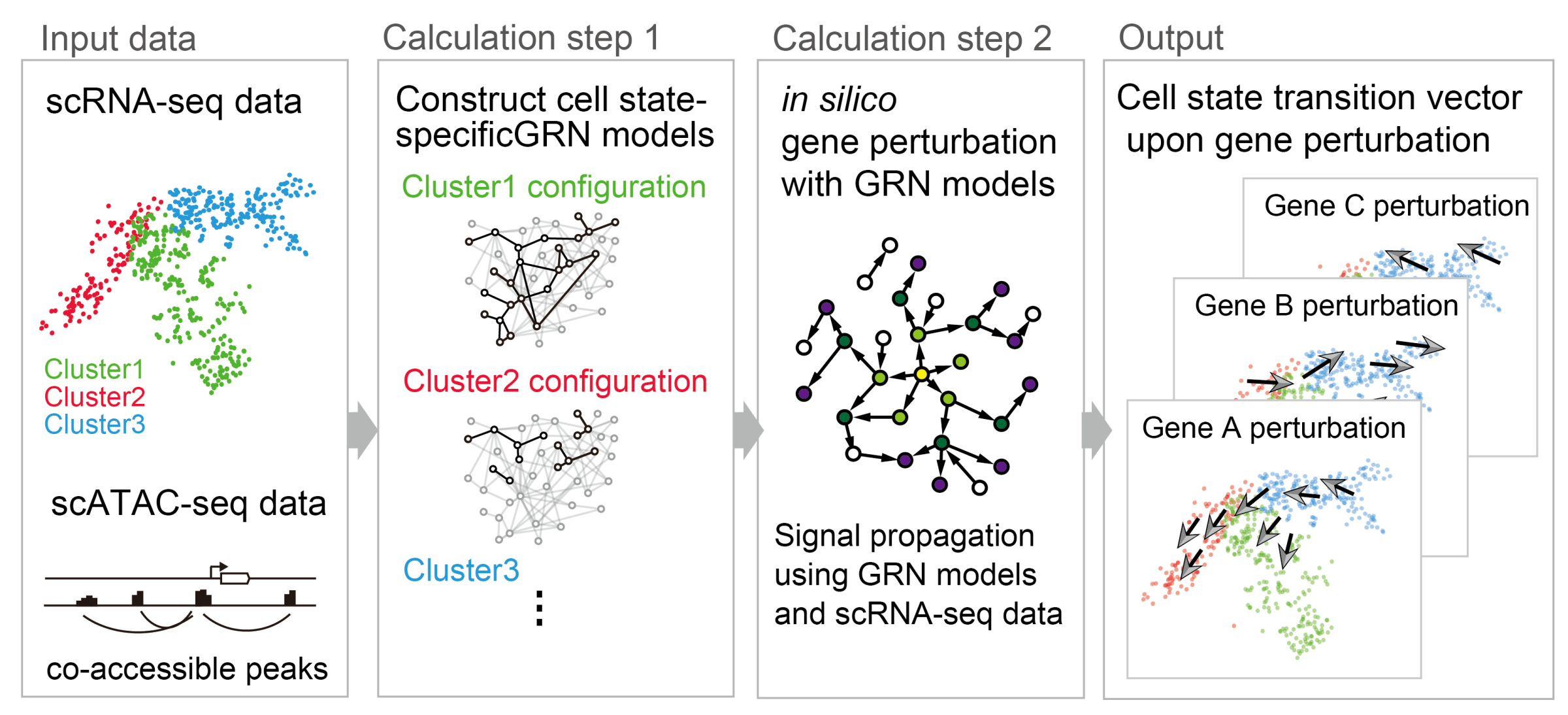
Our bodies are composed of highly diverse cells, the properties of which change dynamically during development and disease. We use single-cell omics technologies, which comprehensively measure genetic information at the single-cell level, to elucidate the processes of cellular change. We aim to consider the heterogeneity and uncertainties of each cell. We have recently developed CellOracle, a technology that models cell state-specific gene programs by integrating single-cell RNA-seq data, single-cell ATAC-seq data, DNA binding information of transcription factors, and other information (Figure 1). CellOracle is designed to use gene regulatory models as predictive simulators of cell identity and to predict the effect of gene perturbation on cell differentiation. We have used this approach to study genetic programs during development in model organisms, such as mice and zebrafish. We have also applied CellOracle to study human disease.
For the omics data analysis and in silico research, AI or machine learning methods are vital to discovering patterns in the vast amounts of data and translating these findings into predictive and simulation models of life. Our group will focus on developing custom AI model structures and learning methods by actively incorporating biological knowledge and hypotheses, rather than simply transferring existing general AI methods to life science data. Thus, our research focuses on establishing Biology-Informed Machine Learning methodologies.
Bioengineering and Synthetic biology research using Bioinformatics
To treat disease, we need to manipulate cells to return abnormal cells to their normal state. In regenerative medicine, we need to create cells to replace those lost from disease. Traditional approaches to manipulating biomaterials involve random screening to discover effective conditions or novel substances by chance; however, the potential conditions for manipulating genes and cells are immense. The more complex the manipulation, the less likely it is to be discovered by chance.
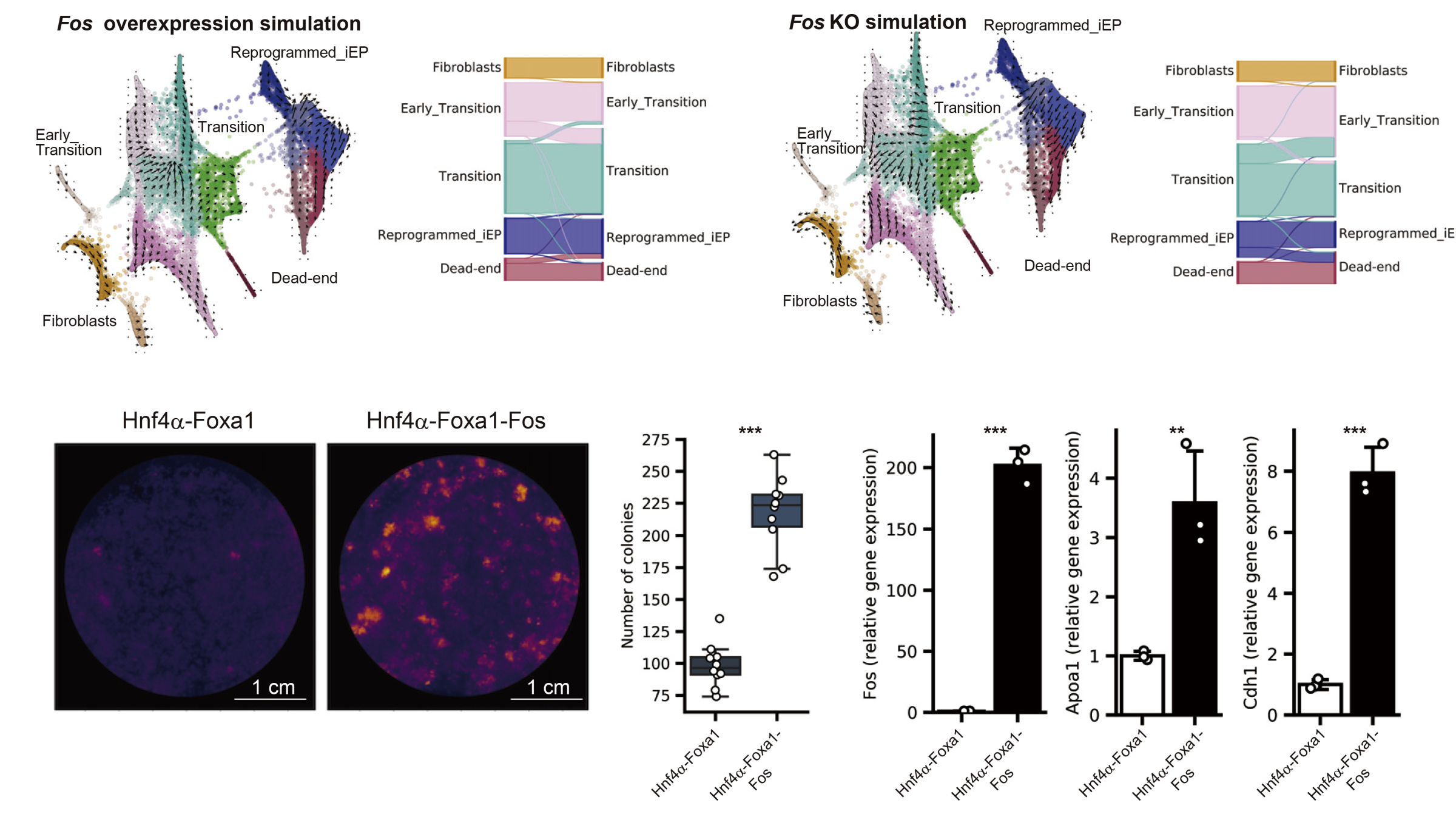
To advance cell manipulation, we believe it is essential to rationally control and design cells based on a systematic understanding and prediction of cellular regulation. We aim to develop a different strategy that does not rely on random discovery. Therefore, we are building AI models and mathematical models that reproduce the function of cells and genes of interest on a computer (in silico), and manipulating cells and genes guided by these in silico simulations. In a recently published paper, we applied in silico analysis techniques to create endoderm progenitor cells, which are the source of the liver and intestines. We identified new key regulatory genes for the induction of endoderm progenitor cells using predictive simulation models (Figure 2). We are also developing DNA engineering technologies to be used in conjunction with in silico prediction technologies.
-
Figure 1: A new method integrating single-cell omics data to reconstruct gene regulatory network models. Our model can be used to perform in silico gene perturbation.
-
Figure 2: Bioengineering of cells guided by in silico predictive models. We identified key regulatory genes in the direct reprogramming process of endoderm progenitor cells.
Staff
- Prof.: Kenji Kamimoto
- Asst. Prof. : Kenji Ito
- SA Asst. Prof. : Shuhei Nozaki (concur.)
- SA Asst. Prof. : Yuki Hagiwara
Website
Publications
1. Single-cell lineage capture across genomic modalities with CellTag-multi reveals fate-specific gene regulatory changes. Jindal, K., et al., Nature Biotechnology (2024) 42, 946–959.
2. Defining cardiac functional recovery in end-stage heart failure at single-cell resolution. Amrute, J.M. et al., Nature Cardiovascular Research. (2023) 2, 399–416.
3. Dissecting cell identity via network inference and in silico gene perturbation. Kamimoto, K.,et al., Nature (2023) 614, 742–751.
4. Gene regulatory network reconfiguration in direct lineage reprogramming. Kamimoto, K., et al., Stem Cell Reports (2023) 18, 1, 97–112
5. The coding and long noncoding single-cell atlas of the developing human fetal striatum. Bocchi, V.D., et al., Science (2021) 1979-372. 10.1126/science.abf5759,
6. Single-cell mapping of lineage and identity in direct reprogramming. Biddy, B.A., et al., Nature (2018) 564, 219–224.
- Home
- Laboratories
- Kamimoto Lab