Yamamoto Lab/Division of Infectious Disease Department of Immunoparasitology
In our Immunoparasitology laboratory, we use the apicomplexan protozoan parasite Toxoplasma gondii as a model for exploring host defense systems and pathogenesis. Our research goal is to elucidate the molecular mechanisms underlying the interface between the host and pathogen.
Toxoplasma gondii is an obligatory intracellular protozoan pathogen that causes lethal toxoplasmosis in humans and animals. One third of the global population is thought to be infected with this pathogen, making it the “most successful parasite.” T. gondii infects virtually all nucleated cells in warm-blooded animals. The parasite forms a special membranous structure called a “parasitophorous vacuole (PV).” The host-parasite interaction takes place through the PV. In response to T. gondii, the host immune system produces inflammatory cytokines such as interleukins, chemokines, and interferons. Interferon-γ (IFN-γ) is the most important host factor for inducing anti-T. gondii responses, which suppress and kill the parasites. One of the main projects in our laboratory is to identify the IFN-γ-induced anti-T. gondii host defense mechanisms involved in innate and adaptive immunity. Recently, we found that IFN-γ-inducible GTPases called GBPs are important for T. gondii PV disruption, and that their function in anti-T. gondii responses requires autophagy proteins; this suggests an unexpected link between IFN-γ-induced immunity and autophagic pathways.
On the other hand, virulent T. gondii suppress IFN-γ-induced host immunity and even manipulate host immune cells to maximize the virulence of the parasite. Another main project in our laboratory is to identify novel virulence mechanisms used by T. gondii. For example, we recently showed that a T. gondii-secreting virulence factor, GRA6, directly activates the host transcription factor NFAT4 to induce chemokines and recruit neutrophils to eradicate the parasite. Thus, our laboratory is focusing on host-parasite interactions via immunoparasitological mechanisms.
-
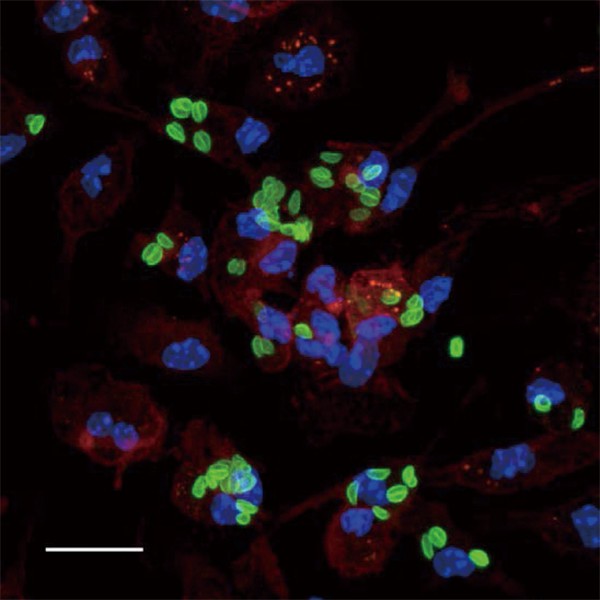
Fig.1. Toxoplasma gondii (green) proliferating inside macrophages (red).
-
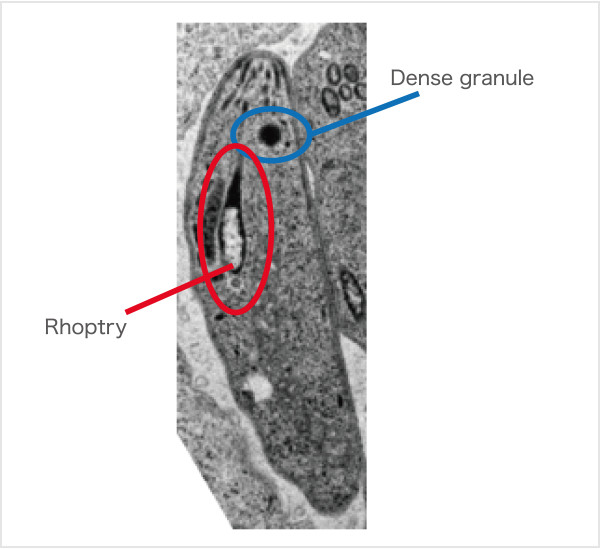
Fig.2. Toxoplasma gondii Pathogenic proteins are secreted from Dense granules and Rhoptry.
-
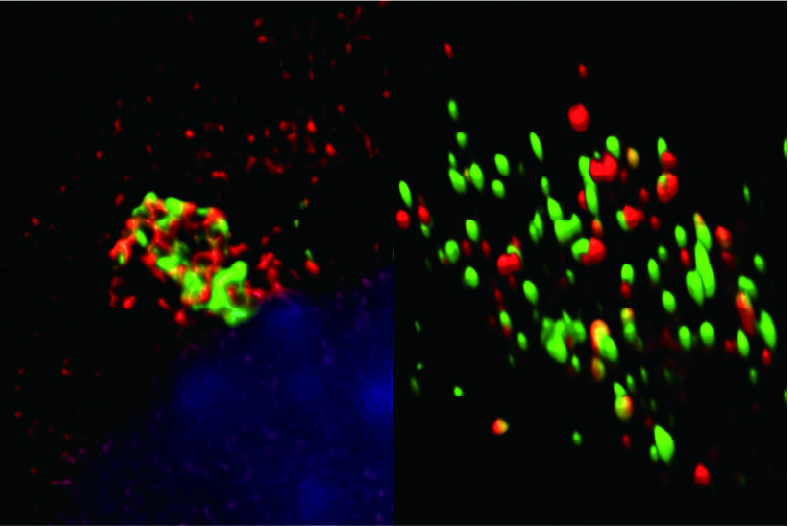
Fig. 3. Gate-16 (red) is required for antimicrobial host defense through cytosolic distribution of GTPase (green). GTPase punctae are colocalized with Gate-16 (right fig.) throughout the cell. However, expression of mutant Gate-16 leads to GTPase aggregation and hampers immune response against pathogens.
Staff
- Prof. : Masahiro Yamamoto
- Assoc. Prof. : Miwa Sasai
- Asst. Prof: Yuta tachibana
- SA Asst. Prof: Ayumi Kuratani
Website
Publications
- (1) Platelet factor 4-induced TH1-Treg polarization suppresses antitumor immunity. Kuratani A., et al. Science. (2024) 386:eadn8608.
(2) Far-East Asian Toxoplasma isolates share ancestry with North and South/Central American recombinant lineages. Ihara F., et al. Nature Commun. (2024) 15:4278.
(3) Uncovering a novel role of PLCβ4 in selectively mediating TCR signaling in CD8+ but not CD4+ T cells. Sasai M., et al. J Exp Med. (2021) 218:e20201763.
(4) CXCR4 regulates Plasmodium development in mouse and human hepatocytes. Bando H, et al. J Exp Med. (2019) 216:1733-1748.
(5) Essential role for GABARAP autophagy proteins in interferon-inducible GTPase-mediated host defense. Sasai M., et al., Nature Immunol. (2017) 18(8):899-910.
(6) Selective and strain-specific NFAT4 activation by the Toxoplasma gondii polymorphic dense granule protein GRA6. Ma J.S., et al., J Exp Med. (2014) 211:2013-32.
(7) A cluster of interferon-γ-inducible p65 GTPases plays a critical role in host defense against Toxoplasma gondii. Yamamoto M., et al., Immunity (2012) 37:302-13.
- Home
- Laboratories
- Yamamoto Lab