Yoshioka Lab/BIKEN Innovative Vaccine Research Alliance Laboratories Vaccine Creation Group
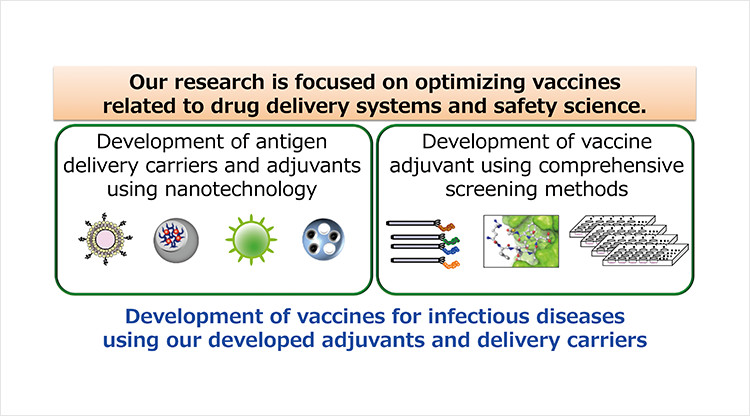
Most protein antigens such as non-living macromolecules or protein-subunit antigens evoke weak or undetectable adaptive immune responses. Therefore, to develop effective vaccines it is necessary to develop vaccine adjuvants and antigen delivery carriers. In addition, to develop optimal (in terms of efficacy and safety) vaccines for clinical application, it is important to understand the mechanism by which vaccines act on the immune system. In this regard, our research is focused on optimizing vaccines through drug delivery systems and safety science. Our specific research projects are:
1) Development of vaccine adjuvants using comprehensive screening methods.
2) Development of antigen delivery carriers and adjuvants using nanotechnology.
3) To use these adjuvants and delivery carriers to develop vaccines for infectious diseases.
Staff
- SA Prof.: Yasuo Yoshioka(concur.)
- SA Assoc. Prof.: Toshiro Hirai (concur.)
Website
Publications
(1) Intranasal immunization with an RBD-hemagglutinin fusion protein harnesses preexisting immunity to enhance antigen-specific responses. Kawai A. et. al. J Clin Invest. (2023)133(23):e166827.
(2) Upregulation of Robo4 expression by SMAD signaling suppresses vascular permeability and mortality in endotoxemia and COVID-19 models. Morita M. et al. Proc Natl Acad Sci USA. (2023)120(3):e2213317120.
(3) Elucidation of the role of nucleolin as a cell surface receptor for nucleic acid-based adjuvants. Kitagawa S. et al. NPJ Vaccines. (2022)7(1):115.
(4) Efficient antigen delivery by dendritic cell-targeting peptide via nucleolin confers superior vaccine effects in mice. Matsuda T. et al. iScience.(2022)25(11):105324.
(5) SARS-CoV-2 disrupts respiratory vascular barriers by suppressing Claudin-5 expression. Hashimoto R. et al. Sci Adv. (2022)8(38):eabo6783.
(6) Synergistic effect of non-neutralizing antibodies and interferon-γ for cross-protection against influenza. Shibuya M. et al. iScience. (2021)24(10):103131.
(7) The Potential of Neuraminidase as an Antigen for Nasal Vaccines To Increase Cross-Protection against Influenza Viruses. Kawai A et al. J Virol. (2021)95(20):e0118021.
(8) Neutrophil-Mediated Lung Injury Both via TLR2-Dependent Production of IL-1α and IL-12 p40, and TLR2-Independent CARDS Toxin after Mycoplasma pneumoniae Infection in Mice. Tamiya S. et al. Microbiol Spectr. (2021)9(3):e0158821.
(9) Murine Cross-Reactive Nonneutralizing Polyclonal IgG1 Antibodies Induced by Influenza Vaccine Inhibit the Cross-Protective Effect of IgG2 against Heterologous Virus in Mice. Shibuya M. et al. J Virol.(2020)94(12):e00323-20.
(10) Lipid Nanoparticles Potentiate CpG-Oligodeoxynucleotide-Based Vaccine for Influenza Virus. Shirai S. et al. Front Immunol.(2020)10:3018.
(11) Intracellular trafficking of particles inside endosomal vesicles is regulated by particle size. Aoyama M. et al. J Control Release.(2017)260:183-193.
(12) Distribution of Silver Nanoparticles to Breast Milk and Their Biological Effects on Breast-Fed Offspring Mice. Morishita Y. et al. ACS Nano. (2016)10(9):8180-91.
(13) Metal nanoparticles in the presence of lipopolysaccharides trigger the onset of metal allergy in mice. Hirai T. et al. Nat Nanotechnol. (2016)11(9):808-16.
- Home
- Laboratories
- Yoshioka Lab