In our immunoparasitology laboratory, the apicomplexan protozoan parasite called Toxoplasma gondii serves as a research model for analyzing host-to-pathogen interactions.
1) What is Toxoplasma?
T. gondii is a eukaryotic pathogen that causes life-threatening toxoplasmosis, a condition that includes encephalitis in immunocompromised individuals such as those who are being treated by chemotherapy or have AIDS. It also causes congenital diseases on primary infection during pregnancy in humans and animals. It is an obligate intracellular eukaryotic parasite that can proliferate exclusively inside a parasitophorous vacuole, which is formed during host–cell invasion. Taxonomically, T. gondii belongs to the phylum of Apicomplexa, which is characterized by the presence of an apical complex with secretory organelles such as conoids, micronemes, and rhoptries (Figure 1A). The rhoptries, which are large bulb-shaped organelles, contain a variety of proteins that are secreted into the host cytoplasm or in the forming parasitophorous vacuole during parasite entry. These proteins then co-opt the host cell for parasite growth and survival (Figure 1B and C).
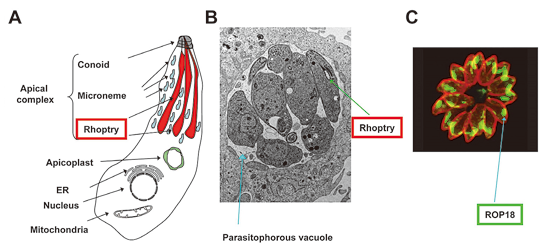
Figure 1 Toxoplasma gondii and the rhophry protein
(A)A schematic figure of T. gondii. The apical complex includes rhoptries (red).
(B)An electron microscopic picture of T. gondii residing in a parasitophorous vacuole (blue) in an infected host cell.
(C)Immunofluorescent picture of T. gondii to detect a rhoptry protein ROP18.
2) Analysis of host cell-autonomous innate immunity against T. gondii.
Interferon-γ (IFN-γ) is essential for host defense against intracellular pathogens. When innate immune cells are stimulated by IFN-γ, they up-regulate ~2000 effector genes such as immunity-related GTPases, including the p65 guanylate-binding protein (GBP) family genes. We showed that a cluster of GBP genes is required for host cellular immunity against T. gondii by first generating mice that lack all six GBP genes on chromosome 3 (Gbpchr3) by targeted chromosome engineering. The mice lacking Gbpchr3 were highly susceptible to T. gondii infection, resulting in increased parasite burden in immune organs. Furthermore, Gbpchr3-deleted macrophages were not able to induce IFN-γ-mediated suppression of intracellular T. gondii growth. Although the IFN-γ-induced oxidative response and the localization of autophagy protein Atg4b were normal, deletion of Gbpchr3 impaired the recruitment of IFN-γ-inducible p47 GTPase Irgb6 to the parasitophorous vacuole. In addition, some members of Gbpchr3 restored the protective response against T. gondii in Gbpchr3-deleted cells. Our results suggest that the Gbpchr3 proteins redundantly play a pivotal role in anti-T. gondii host defense by controlling IFN¬-γ-mediated Irgb6-dependent cellular innate immunity (Figure 2).
GBPs also participate in anti-bacterial responses via autophagy. An essential autophagy protein Atg5 was previously shown to play a critical role in cell-autonomous immunity against T. gondii. However, the involvement of other autophagy proteins remains unknown. Here, we show that some essential autophagy proteins, but not all, participate in anti-T. gondii cellular immunity by recruiting IFN-γ-inducible GTPases. In Atg7- or Atg16L1-deficient cells, IFN-γ-induced suppression of T. gondii proliferation and recruitment of the IRG Irgb6 and GBPs are profoundly impaired. However, cells lacking the other essential autophagy proteins Atg9a and Atg14 can mediate the anti-T. gondii response and recruit Irgb6 and GBPs to the parasites. Thus, mouse autophagy components Atg7 and Atg16L1, but not Atg9a and Atg14, participate in the IFN-γ-induced recruitment of immunity-related GTPases to the intracellular pathogen (Figure 2).
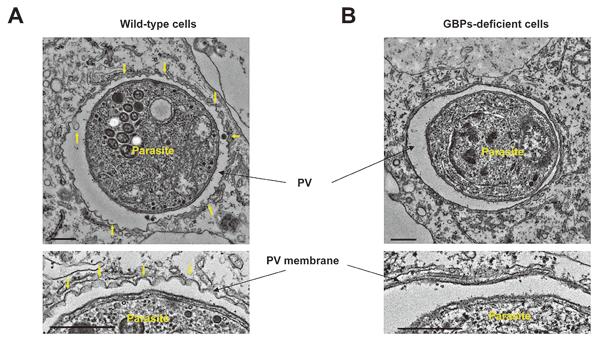
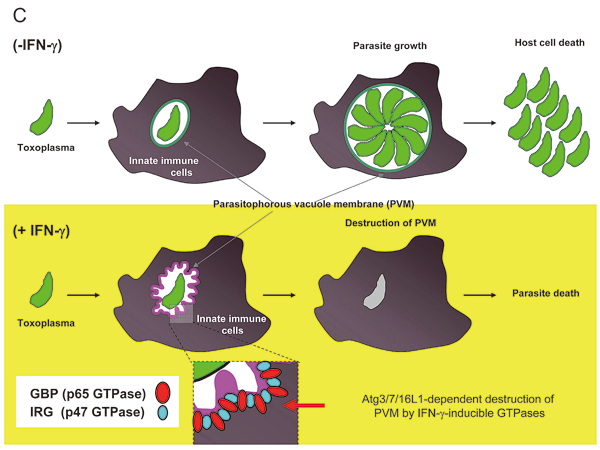
Figure 2 Role of IFN-γ-induced GBPs in anti-T. gondii cell-autonomous immunity
(A) Structure of PV membrane was destroyed in IFN-γ-treated wild-type cells, but not in(B)GBPs-deficient cells.
Major publications
- Ohshima J, Lee Y, Sasai M, Saitoh T, Ma JS, Kamiyama N, Matsuura Y, Pann-Ghill S, Hayashi M, Ebisu S, Takeda K, Akira S, Yamamoto M. Role of the mouse and human autophagy proteins in IFN-γ-induced cell-autonomous responses against Toxoplasma gondii. J Immunol. 2014, 192: 3328-3335.
- Yamamoto M, Okuyama M, Ma JS, Kimura T, Kamiyama N, Saiga H, Ohshima J, Sasai M, Kayama H, Okamoto T, Huang DS, Soldati-Favre D, Horie K, Takeda J, Takeda K. A cluster of interferon-γ-inducible p65 GTPases plays a critical role in host defense against Toxoplasma gondii. Immunity. 2012, 37:302-313.
- Yamamoto M, Ma JS, Mueller C, Kamiyama N, Saiga H, Kubo E, Kimura T, Okamoto T, Okuyama M, Kayama H, Nagamune K, Takashima S, Matsuura Y, Soldati-Favre D, Takeda K. ATF6β is a host cellular target of the Toxoplasma gondii virulence factor ROP18. J Exp Med. 2011, 208:1533-1546.
- Yamamoto M, Standley DM, Takashima S, Saiga H, Okuyama M, Kayama H, Kubo E, Ito H, Takaura M, Matsuda T, Soldati-Favre D, Takeda K. A single polymorphic amino acid on Toxoplasma gondii kinase ROP16 determines the direct and strain-specific activation of Stat3. J Exp Med. 2009, 206: 2747-2760.