We are studying the eukaryotic cell cycle to understand the mechanisms underlying the observed chromosomal instability in cancer cells. Many human cancer cells exhibit mitotic defects (such as centrosome aberrations, abnormal spindle formation, and chromosome missegregation). The resulting chromosomal instability is a major cause of malignant tumor progression. Our research focuses on functional analyses of the Ser/Thr kinases Lats (large tumor suppressor) and GAK (cyclin G-associated kinase). These kinases localize to the centrosome, regulate mitotic progression in response to DNA damage, and cause chromosome instability when their functions are disrupted. Both Lats (Lats1 and Lats2) and GAK form complexes with Mdm2. In turn, Mdm2 controls the stability of p53, which is a transcriptional regulator of the Lats2, cyclin G1, and Mdm2 genes. Thus, the Lats and GAK complexes have intimate correlations in their regulation of cell cycle (Fig. 1).
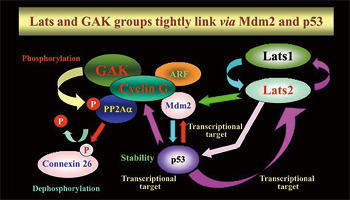
Fig. 1: The functions of the Lats and GAK complexes
correlate closely.
Our research subjects are as follows:
(1) Lats Group
Lats1 and Lats2, which belong to the Lats kinase family, are highly conserved across species and localize to the centrosome during the cell cycle. Lats1/2 plays an important role in the cell cycle checkpoint and the Hippo pathway that mainly regulates organ size and cell growth. In particular, Lats2 binds to Mdm2, thereby inhibiting its E3 ligase activity and activating p53. Thereafter, p53 rapidly and selectively upregulates Lats2 expression in G2/M cells. This positive feedback loop constitutes a novel checkpoint that plays a critical role in the maintenance of proper chromosome numbers (Aylon et al., Gene Dev., 2006).
We have discovered the following: (A) LATS2 knockout mice are embryonic lethal, which indicates the essential role of Lats2 in the development and differentiation of mammalian germ cells. (B) Lats2-/- mouse embryonic fibroblasts (MEF) display mitotic defects such as centrosome fragmentation, abnormal chromosome segregation, and aberrant cytokinesis (Yabuta et al., J. Biol. Chem., 2007). (C) MEFs from mice in which Lats1 is knocked out by disrupting its N-terminal region (Lats1ΔN/ΔN) display downregulation of Lats2 mRNA expression and protein accumulation of Yap, a downstream factor of Lats2 in the Hippo pathway (Ref. 4). Moreover, the MEFs also show mitotic defects such as centrosome amplification, chromosome missegregation (Fig. 2), cytokinesis failure, and anchorage-independent growth (Fig. 3). These results indicate the essential role that Lats2 and Lats1 play in proper M phase progression and chromosome stability. (D) Lats2 mediates two novel signaling pathways: CLP (Chk1/2—Lats2—P-body) and ALB (Aurora-A—Lats1/2—Aurora-B). In the CLP pathway, Lats2 phosphorylates 14-3-3 for processing body (P-body) formation downstream of Chk1 in response to DNA damage (Okada et al., J. Cell Sci., 2011). Moreover, under severe DNA damage, Lats2 is phosphorylated both by Chk1 and itself and fully activated, thereby inducing caspase-dependent apoptosis through Lats2-mediated phosphorylation of p21/CDKN1A and subsequent protein degradation of p21 (Ref. 2, Fig. 4). In the ALB pathway, Lats2 is phosphorylated by Aurora-A. Phosphorylated Lats2 localizes to the chromosomes and the central spindle, and regulates Lats1 and Aurora-B, thereby executing proper chromosomal segregation during mitosis (Yabuta et al., Cell Cycle, 2011). (D) Lats2 phosphorylates and regulates ASPP1/p53 complexes, thereby inducing apoptosis in malignant tumor cells with aneuploidy and polyploidy (Aylon et al., Genes Dev., 2010). Moreover, Lats2 phosphorylates the transcription factor Snail, thereby regulating the epithelial-mesenchymal transition (EMT) (Zhang et al., EMBO J., 2012).
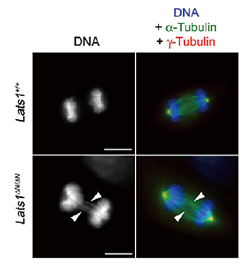
| Fig. 2: | Lats1ΔN/ΔNMEFs exhibit chromosomal missegregation during anaphase. |
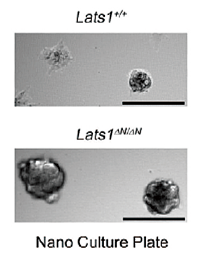
| Fig. 3: | Lats1ΔN/ΔNMEFs exhibit anchorage-independent growth. |

| Fig. 4: | Activation of Lats2 by Chk1-mediated and auto-phosphorylation induces apoptosis (red). |
(2) GAK Group
GAK is an association partner of clathrin heavy chain (CHC) and is essential for clathrin-mediated membrane trafficking. Unlike neuron-specific auxilin, which plays a similar role in neural cells, GAK has a kinase domain whose function was unclear. We have discovered the following: (a) GAK forms a complex with PP2A B’γ and cyclin G (cyclin G1 and cyclin G2), which regulates the dephosphorylation activity of PP2A (Ref5, Fig. 5); (b) GAK localizes to the cytoplasm and the nucleus, where it has two additional functions (i.e., maintenance of proper centrosome maturation and mitotic chromosome congression; Shimizu et al., J. Cell Sci., 2009); and (c) GAK-kd-/- mice exhibit neonatal lethality with pulmonary dysfunction (Fig. 6). Since the EGFR inhibitor Gefitinib (Iressa) also efficiently inhibits the kinase activity of GAK, the side effects of Gefitinib, including interstitial pneumonia, may be due to the inhibition of GAK (Tabara et al., PLoS One, 2011). (d) Cyclin G2 knockout (Ccng2-/-) mice are born and develop normally. However, when Ccng2-/- MEFs are irradiated with ionizing radiation (IR), their dephosphorylation of γH2AX after DNA damage (i.e., the cancellation of the DNA damage response) is severely retarded (Ref. 3, Fig. 7). These results suggest that Cyclin G2 regulates the dephosphorylation of γH2AX by recruiting PP2A after the completion of DNA repair, thereby causing the cells to recover from DNA damage checkpoint-dependent cell cycle arrest.

| Fig. 5: | T104 of PP2A B’γ by GAK is an important phosphorylation site for the activity of PP2A complex. |
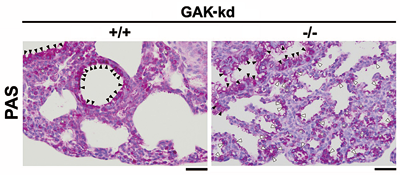
| Fig. 6: | Severe defects of fetal lung maturity in GAK-kd-/-mice. |
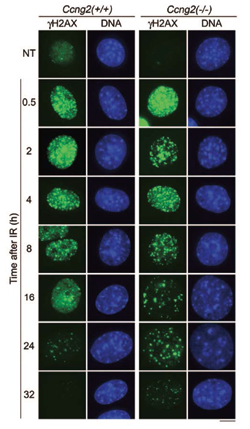
| Fig. 7; | Cyclin G2 knockout cells show that the dephosphorylation of γH2AX is retarded after IR irradiation (24 h and 32 h, green). |