Cells are equipped with the signal transduction system, which enables them to respond appropriately to the surrounding environment, such as stimulation with various hormones/growth factors and physical interactions with other cells or the extracellular matrix. Malfunction in the signaling system is responsible for various human diseases. A typical pathogenic consequence of such malfunction is the abnormal proliferation of cancer cells. In our laboratory, we are investigating the intracellular signaling that regulates the proliferation, differentiation, and motility of cells at various levels, ranging from molecules to organisms (nematodes and mice). The present main research interests are (1) oxidative stress signaling by reversible oxidation of proteins, and (2) regulation of intracellular magnesium levels and signal transduction.
(1) Oxidative stress signaling by reversible oxidation of proteins
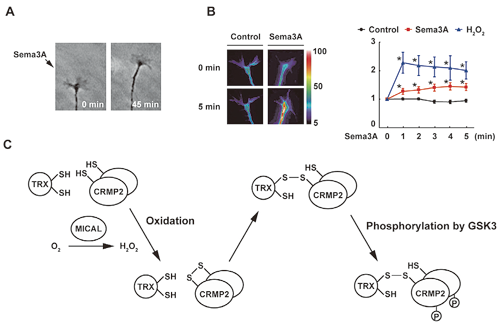
We discovered that thioredoxin-related protein nucleoredoxin (NRX) is a novel regulator of Wnt signaling, which plays important roles in early development and oncogenesis. NRX binds directly to Wnt signal transducer Dishevelled (Dvl) and inhibits its function. Interestingly, the NRX-Dvl interaction is negatively regulated by the formation of intramolecular S–S bonds in NRX. Thus, NRX regulates Wnt signaling in a redox-dependent manner. We also developed a novel method to search for proteins forming S–S bonds in cells by using thioredoxin mutants. With this method, we identified several proteins such as CRMP2, which functions in Semaphorin signaling. Semaphorin treatment stimulates H2O2 generation and CRMP2 oxidation, resulting in the formation of homodimers linked with S–S bonds. This oxidation of CRMP2 mediates the repulsive axon guidance induced by Semaphorin stimulation (Fig. 1).
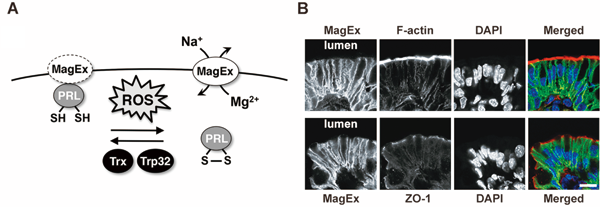
(2) Regulation of intracellular magnesium levels and signal transduction
We found that PRL, a tyrosine phosphatase with unknown function, is a novel S–S bond-containing protein. It has been reported that PRL is consistently overexpressed in various human metastatic cancers and can promote experimental metastasis in mice. We searched for novel PRL-binding proteins and identified Magnesium-Exporting protein (MagEx). MagEx regulates the intracellular Mg2+ levels by exporting Mg2+. This function of MagEx is inhibited by the interaction with PRL. Since this PRL-MagEx interaction is dependent on the redox state of PRL, PRL functions as a redox switch that regulates intracellular Mg2+ levels. Endogenous MagEx is highly expressed in the intestinal epithelia and specifically localizes in their basolateral membrane and causes hypomagnesemia. Gene disruption of MagEx in mice severely impairs the ability of their intestines to absorb magnesium. In addition, MagEx is also highly expressed in the epithelial cells that form enamel (ameloblasts). Indeed, MagEx-knockout mice show symptoms of amelogenesis imperfecta (Fig. 2). These results demonstrate the importance of MagEx in the control of systemic and local magnesium levels, the dysfunction of which results in the development of various diseases.