We are studying the mechanisms of cell growth and differentiation that involve growth factors and adhesion molecules presented at cell–cell contact sites. In particular, we are focusing on the mode of action of HB-EGF, a membrane-anchored EGF family of growth factors, and the tetraspanin family, which are the molecules to which HB-EGF binds. These proteins function in morphogenesis and tissue maintenance and repair by regulating cell proliferation, migration and adhesion. They are also involved in the growth, invasion and metastasis of cancer cells.
1) Mode of action of HB-EGF
HB-EGF is a member of the EGF family of growth factors, and binds to and activates EGFR and ErbB4. It is synthesized as proHB-EGF, a membrane-anchored precursor protein, and is cleaved on the cell surface to yield the soluble growth factor (sHB-EGF). The conversion of proHB-EGF into the soluble form is critical for the activity of this growth factor, and therefore this process is tightly regulated. HB-EGF is secreted by various tissues and cells and functions in several physiological processes. For example, it maintains heart muscle function, suppresses cell proliferation in heart valve and lung alveolar development, promotes cell migration in wound healing and eyelid closure, supports blastocyst attachment to the uterus during implantation, and promotes cell proliferation in skin hyperplasia. ProHB-EGF is not only a precursor of the soluble form, it is also a biologically active molecule that regulates the growth of neighboring cells in a juxtacrine fashion. How is the conversion of the membrane-anchored form into the soluble form regulated? How does HB-EGF function in the manifold physiological processes that are dependent on this molecule? What roles do sHB-EGF and proHB-EGF play in such physiological processes? Do they participate in pathological processes? These questions are currently being analyzed at the molecular level.
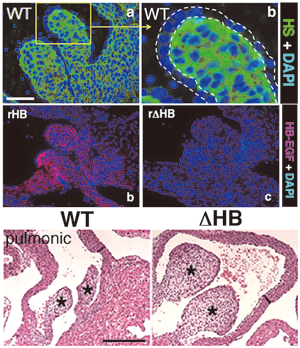
In valve development, HB-EGF is expressed and secreted from endocardial cells. Secreted HB-EGF associates with heparan sulfate proteoglycans (HSPGs) in the valve mesenchyme (upper right). When EGFR on the surface of mesenchymal cells is activated by the HB-EGF-HSPG complex, this receptor transduces inhibitory signals for mesenchymal cell proliferation (lower left). Knock-in mice expressing a mutant HB-EGF that cannot bind to HSPGs (ΔHB), as well as KO mice, develop enlarged cardiac valves characterized by hyperproliferation of mesenchymal cells (lower right) (Iwamoto et al., 2010).
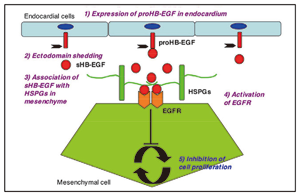
Fig. 1 The role of HB-EGF in heart valve formation. |
|
2) Role of HB-EGF in cancer malignancy
HB-EGF is expressed by cancer cells and/or cancer-derived stroma, and is involved in tumor growth, invasion and metastasis. To develop a novel strategy for cancer therapy, we are analyzing the role of HB-EGF in cancer malignancy.
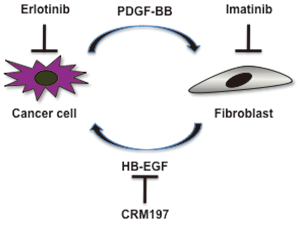
Fig. 2. Cancer cell-stromal fibroblast interactions in the cervix. |
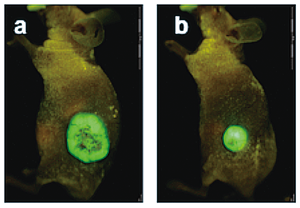
Fig.3. Cancer-derived fibroblasts enhance tumorigenesis of cervical cancer cells in nude mice. Fluorescence imaging of tumors injected cervical cancer cells with fibroblasts (a) or cervical cancer cells alone (b) (Murata et al. 2011). |
3) Development of anti-cancer drugs targeting HB-EGF
We are developing new anti-cancer drugs that target HB-EGF. The pre-clinical and clinical studies of an anti-HB-EGF monoclonal antibody and a non-toxic mutant protein of diphtheria toxin CRM197 are currently in progress.
4) CD9 and the tetraspanin function
CD9 is a member of the tetraspanin superfamily. It is a membrane protein with four transmembrane domains. It associates with proHB-EGF and upregulates proHB-EGF function. CD9 is also involved in cell signaling, growth, motility, and adhesion, and in tumor cell metastasis and sperm–egg fusion. In addition, the Caenorhabditis elegans tetraspanin TSP-15 is essential for the epidermal integrity of the worm. We are analyzing the physiological activity of CD9 and other tetraspanins by using genetically engineered mice or worms (C. elegans) lacking CD9 or other tetraspanins.
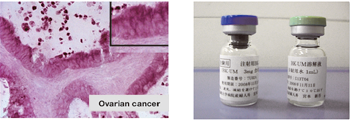
Fig. 4. Enhanced expression of HB-EGF in the ovarian cancer tissue (right) and BK-UM, therapeutics for ovarian cancer under development in our laboratory.