We are working on understanding the molecular mechanisms of hepatitis C virus (HCV) entry, replication, assembly, and pathogenesis, and developing a novel virus vector for gene delivery.
1. Studies on the molecular biology of HCV replication and pathogenesis
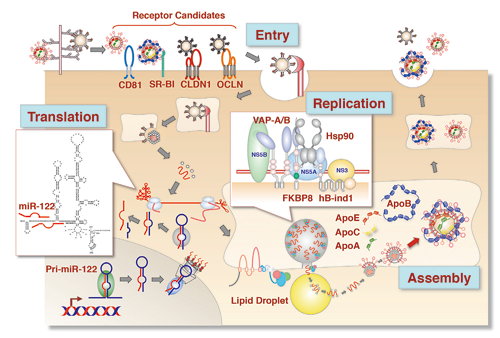
HCV infects over 130 million individuals worldwide and is one of the most common etiologic agents of chronic liver diseases, including liver cirrhosis and hepatocellular carcinoma (HCC). Although novel innovative anti-HCV agents achieve sustained virological responses in chronic hepatitis C patients, drug-resistant viruses emerge frequently. Therefore, host factors that are indispensable for HCV replication are the ideal targets for the development of new broad spectrum therapeutics against chronic hepatitis C that associate with a low possibility of emergence of breakthrough viruses against antiviral drugs. HCV internalizes into cells by endocytosis by interacting with several candidate receptors, including hCD81, SR-BI, Claudin1, and Occludin. After uncoating, the viral RNA is translated into a large precursor polyprotein composed of 3,000 amino acids, which is cleaved by signal peptidase, signal peptide peptidase, and virus-encoded proteases, resulting in at least 10 viral proteins. We have shown by using transgenic mice expressing the HCV core protein, which develop hepatic steatosis and HCC, that knocking out their PA28γ gene inhibits the development of liver steatosis and HCC. This suggests that PA28γ plays a crucial role in the development of the liver failure induced by HCV infection. Furthermore, we have shown that co-chaperones such as FKBP8 and hBind-1 recruit Hsp90 to the HCV replication complex by interacting with NS5A protein, thereby playing an important role in HCV replication. Liver-specific miR-122 enhances the translation of HCV RNA by interacting with its 5’UTR. Several non-hepatic cell lines permit HCV replication when miR-122 is expressed but infectious particles are not produced. This is probably because these cells have low expression levels of apolipoproteins such as ApoB and ApoE, unlike hepatic cell lines such as Huh7 and Hep3B. Indeed, Huh7 cells that are deficient in both ApoB and ApoE genes (DKO cells) exhibit severely impaired infectious particle formation, while exogenous expression of not only ApoE but also other apolipoproteins (including ApoA1, ApoA2, ApoC1, ApoC2, and ApoC3) rescues particle formation. This suggests that ApoA, ApoC and ApoE participate in HCV assembly in a redundant fashion. HCV belongs to the family of Flaviviridae, which includes flaviviruses such as Japanese encephalitis virus (JEV). Since there is a robust cell culture system and a small animal model for JEV, we are also investigating the replication and pathogenesis of JEV as a surrogate model for HCV.
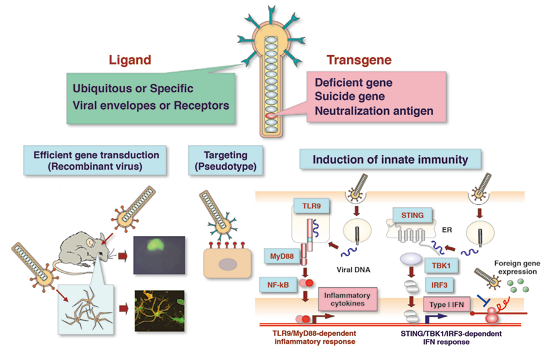
2. Development of baculoviral vectors
Viral vectors are essential tools for studies on replication-deficient viral infectious diseases such as HCV. The development of novel viral vectors is also essential for future gene therapy. We are working on the baculovirus Autographa californica nucleopolyhedrovirus (AcNPV) to develop it into a versatile viral vector for gene delivery not only in vitro but also in vivo. AcNPV is an insect virus with a 134 kb double-stranded circular DNA genome. Due to its strong promoters, baculovirus is commonly used for the large-scale production of recombinant protein in insect cells. Baculovirus is also capable of entering a variety of mammalian cells and expressing foreign genes under the control of mammalian promoters without replicating its viral genome. Therefore, baculovirus is a useful viral vector, not only for the abundant expression of foreign genes in insect cells but also for efficient gene delivery to mammalian cells. AcNPV has a number of unique beneficial properties as a viral vector, including a large capacity for foreign gene incorporation, easy manipulation, and replication competence in insect cells combined with incompetence in mammalian cells. Therefore, it has a significantly lower likelihood of generating replication-competent revertants that express baculoviral gene products (which can lead to harmful immune responses against mammalian cells) than other viral vectors that are currently in use. Furthermore, intranasal inoculation with AcNPV induces a strong innate immune response that protects mice from lethal challenges of influenza viruses. We have shown that internalization of viral DNA by membrane fusion of the envelope glycoprotein is required to induce inflammatory cytokines and type I IFN via the TLR9-dependent and -independent pathway, respectively. Moreover, the induction of IFN-β through the STING/TBK1/IRF3 pathway attenuates the transgene expression in mammalian cells. Thus, innate immune responses induced by infection with AcNPV attenuate its transgene expression. This characteristic means that AcNPV may be useful for selective gene transduction into cells whose ability to evoke innate immunity is impaired by infection with various viruses.