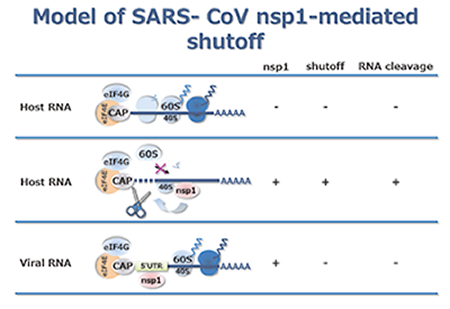
Our group studies the molecular biology and host cell–virus interactions of Severe Acute Respiratory syndrome (SARS) coronavirus (SARS-CoV). SARS-CoV is the etiological agent of a newly-emerged human respiratory disease that originated in southern China in 2002 and spread worldwide in the 2003 epidemic. Coronavirus is an enveloped virus that carries a long, single-stranded, positive-sense genomic RNA. On infection, the genomic RNA is translated to produce two large polyproteins that are then proteolytically processed by two viral proteinases into 16 mature viral proteins called nsp1 to nsp16. Currently, our group is studying the effect of SARS-CoV nsp1 protein on host cells. SARS-CoV nsp1 protein inhibits host gene expression by first binding to the 40S ribosome and then inactivating the translational machinery. In addition, the 40S ribosome-bound nsp1 induces RNA cleavage of host mRNA. The nsp1 protein also binds to the 5’untranslated region (UTR) of SARS-CoV RNA, which confers resistance to the nsp1-mediated shutoff of translation. Our group is also studying various SARS-CoV–host cell interactions to understand SARS-CoV pathogenesis at the molecular level. More recently, another coronavirus called the Middle East respiratory syndrome coronavirus (MERS-CoV) emerged in the Middle East in April of 2012. It too causes acute respiratory distress syndrome. Our group is presently studying the molecular biology and host cell–virus interactions of MERS-CoV.