Research Projects
Malaria is a serious threat to global human health. More than 40% of the world’s population lives in malaria-endemic areas, and two million people die from the disease annually. Controlling malaria has become more challenging since the emergence of drug-resistant malaria parasites. This stresses the need for novel drug-targeting strategies and an effective malaria vaccine. Our department focuses on both malaria vaccine and anti-malarial drug development. We also seek to understand the mechanisms used by the malaria parasite to survive in the host.
(1) Development of a recombinant vaccine based on the malaria protein SERA
We are developing a malaria vaccine based on SE36, a recombinant protein that corresponds to the N-terminal amino acid sequence present in the serine repeat antigen (SERA) 5 of malaria parasites (Ref. 1, 2). An epidemiological study of children living in a malaria-endemic area in Uganda revealed that the children with high antibody titers against the N-terminal domain of SERA5 did not have fever. There was a clear negative correlation between blood parasitemia and anti-SERA IgG antibody titers. In collaboration with The Research Foundation for Microbial Diseases of Osaka University, we produced the GMP-grade malaria vaccine candidate BK-SE36, which is composed of SE36 and aluminum hydroxide gel. We conducted a Phase Ib clinical trial of BK-SE36 with 140 volunteers in Uganda between 2010 and 2011. The safety of BK-SE36 was demonstrated in Stage 1 of the trial with Ugandan male and female adults and in the following Stage 2 with Ugandan children aged 6–20 years. We followed up the volunteers for 1 year after vaccination and observed their malaria infection. An efficacy rate of 72% (p=0.003) against symptomatic malaria was obtained when BK-SE36 vaccinees were compared to the control cohort (Ref. 2). Although the polymorphism of malaria antigens often complicates malaria vaccine development, SERA genes do not show antigenic variation (Aoki; JBC, 2002) and the polymorphism of the antigen is also limited (Ref. 4). This suggests that this vaccine may be effective against a wide variety of malaria parasite strains. We will conduct further clinical trials and proceed to the development of the vaccine for use in malaria-endemic areas.

Fig. 1. Patients at Apac hospital (Uganda). Of the patients waiting at the Out-patient Department of Apac Hospital in Northern Uganda, majority are children under 5 years old. |
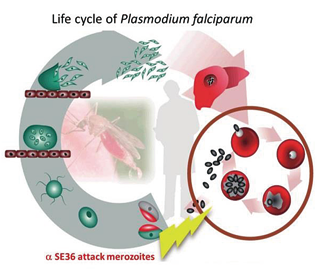
Fig. 2. Life cycle of Plasmodium falciparum and the action point of anti-SE36 antibody induced by SE36 malaria vaccine. |
Fig. 3. The SE36 malaria vaccine for clinical trials is produced under Good Manufacturing Practices (GMP) conditions at the Kanonji Institute of The Research Foundation for Microbial Diseases of Osaka University. |
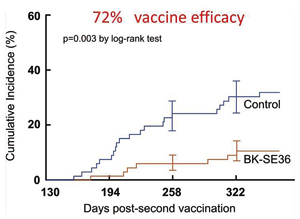
Fig. 4. Kaplan-Meier plot of P. falciparum malaria episodes after vaccination. |
(2) Approaches to understanding the evolution of malaria parasites
Malaria parasites have many unique features, such as a highly restricted host range, a complex life cycle, and host immune evasion systems. To understand the molecular basis of those features, we utilized several genetic approaches, including nuclear and organelle genome analyses, as well as population genetic analyses of the antigen-coding genes (Ref. 4, 5). The findings from these studies will be useful for vaccine development.

Fig. 5. Evolutionary tree of malaria parasites inferred from 30 protein-encoding genes from the apicoplast genome. Four human parasites do not show a monophyletic relationship revealing host-switching during parasite evolution.