In the past, naturally mutated animals were used to elucidate the mechanisms of various diseases. In the "post-genome project era", however, genetically manipulated animals play a key role in such investigations as they are highly useful for providing disease models. Our laboratory, in collaboration with the Animal Resource Center for Infectious Diseases, is researching the mammalian reproductive system by genetically manipulating mice (http://www.egr.biken.osaka-u.ac.jp/).
Research Projects
We were the first group in the world to produce a genetically altered “green mouse” that glows in the dark. These GFP-expressing mice are highly useful for many fields of research, such as stem cell transplantation and regeneration. By utilizing these animals, we showed that it was possible to label sperm with fluorescent proteins and to visualize the fertilization process (Fig. 1–3). By utilizing gene knockout (KO) technology, we also showed that testis-specific chaperones (CLGN, CALR3, and PDILT) are required for the quality control of the sperm membrane protein ADAM3 in the endoplasmic reticulum. The Pdilt KO mice were found to be male infertile because their spermatozoa lack ADAM3 and cannot migrate through the utero-tubal junction (Fig. 2, #5). The testicular germ cell-specific GPI-anchored protein TEX101 was also found to be required for the presence of ADAM3 (#3). Finally, we were successful in visualizing the moment of sperm-egg fusion by using transgenic mouse lines in which IZUMO1 was tagged with a fluorescent protein (Fig. 3, #4).
We are also seeking to invent new technologies for novel investigations in the field of reproduction. By transducing blastocysts with lentiviral vectors, we developed the placenta-specific gene manipulation method (Nat Biotechnol, 2007). Expression of soluble FLT1 in the placenta generates preeclampsia model mice (PNAS, 2011). We established rat embryonic stem (ES) cells and used them to generate Mouse↔Rat chimeric animals (Fig. 4, Genes Cells, 2011). These chimeric animals may be useful for generating various organs from ES or iPS cells. To examine the role(s) of miRNA, we have analyzed miRNA KO mice (#2). We also successfully generated genetically modified animals (mice and rats) by using the CRISPR/Cas system (#1). Our recent interest is to use the CRISPR/Cas system to generate gene-modified animals for studying fertilization, implantation, and placentation.
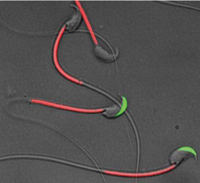
Fig. 1) A transgenic mouse spermatozoa labeled with green fluorescent protein (GFP) in their acrosome and red fluorescent protein (RFP) in their mitochondria. Spermatozoa lose green fluorescence after acrosome reaction.
|
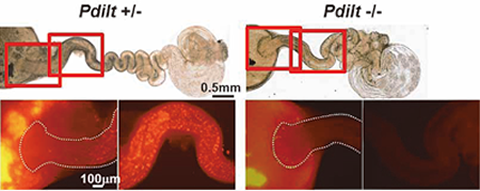
Fig. 2) Pdilt -/- mouse spermatozoa have impaired ability to migrate from uterus into the oviduct.
|
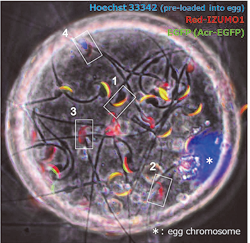
Fig. 3) Red-IZUMO1 transgenic spermatozoa bind onto oocyte plasma membrane. Acrosome reacted spermatozoa indicate altered distribution of IZUMO1 by red fluorescence (2 or 3, GFP negative). Fused sperm (4) shows specific pattern of fluorescent lost.
|
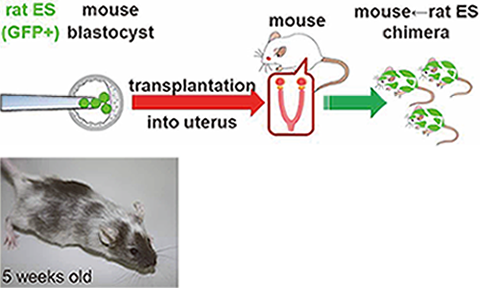
Fig. 4) Mouse↔Rat chimeric animals are generated by injecting rat ES cells
|
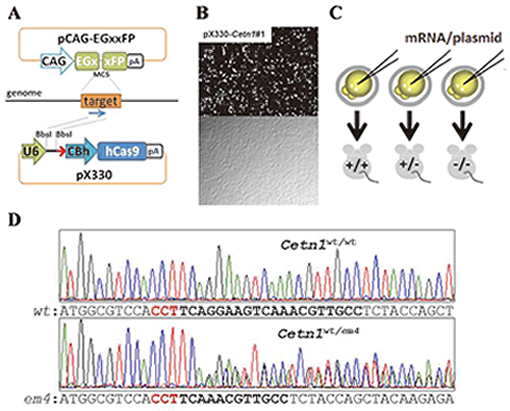
Fig. 5. A. pCAG-EGxxFP target plasmid contains overlapping 5’ and 3’ EGFP fragments under the CAG promoter. pX330 plasmid expresses both guide RNA (gRNA) and hCas9 endonuclease.
B.The efficiency of DSB mediated homology dependent repair was validated by observing EGFP fluorescence 48 hrs after the transfection (top; fluorescent field , bottom; bright field).
C. To generate gene modified mice, fertilized eggs were injected with mRNAs coding hCas9 and gRNA into cytoplasm or pX330 plasmid into pronuclei.
D. CRISPR/Cas9 mediated Cetn1 mutations observed in founder mice. Representative Cetn1 genomic sequences from founder mice (top; wild-type (WT), bottom; heterozygous 8 bp deletion (em4)).