Main text
We study the innate immune system, which is an evolutionally conserved host defense mechanism against microbes. Pattern-recognition receptors (PRRs) play a critical role in the induction of innate immunity. PRRs detect various microorganisms ranging from bacteria to fungi, protozoa and viruses, and induce the production of inflammatory factors such as cytokines and interferons. However, PRRs also detect environmental irritants and host-derived stimulatory factors, and can cause the development of inflammatory diseases. Thus, the innate immune system can be a double-edged sword for the host. To gain a deeper understanding of the innate immune system, we have examined a mechanism that regulates PRR-mediated innate immune responses and a novel function of innate immune cells.
1. Microtubule-dependent activation of the NLRP3-inflammasome
NLRP3 forms an inflammasome with its adaptor ASC, and its excessive activation can cause inflammatory diseases. However, little was known about the mechanisms that control the assembly of the inflammasome complex. We found that microtubules mediate the assembly of the NLRP3 inflammasome (Misawa T et al., Nat Immunol, 2013). Thus, inducers of the NLRP3 inflammasome cause aberrant mitochondrial homeostasis, which in turn diminishes the concentration of the coenzyme NAD. This then inactivates the NAD-dependent protein α-tubulin deacetylase sirtuin 2, thereby causing acetylated α-tubulin to accumulate. The acetylated α-tubulin mediates the dynein-dependent transport of mitochondria and the subsequent apposition of ASC on mitochondria to NLRP3 on the endoplasmic reticulum. Therefore, the activation of the entire NLRP3 inflammasome requires both the direct activation of NLRP3 and the creation by microtubules of optimal sites for signal transduction (Figure 1).
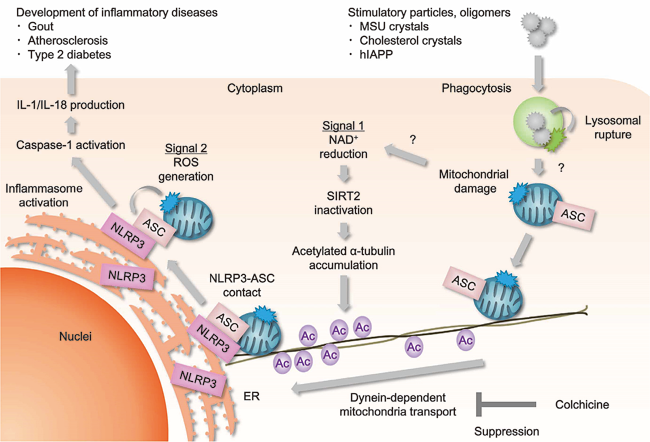
Figure 1. Activation of the NLRP3-inflammasome.
2. Involvement of cathepsin D in the immunomodulatory effects of synthetic double-stranded RNA
RIG-I-like receptors (RLRs) detect double-stranded (ds) RNA and induce antiviral immune responses. RLRs also mediate the adjuvant effect of polyinosinic-polycytidylic acid (poly IC), a synthetic dsRNA. We revealed the mechanisms of the poly IC-induced innate immune responses (Zou J et al., Immunity, 2013). Poly IC is taken up by dendritic cells (DCs) and induces lysosomal destabilization, which causes the release of cathepsin D into the cytoplasm from the lysosome. Cathepsin D then interacts with IPS-1, an adaptor molecule for RLRs. This interaction activates the RLR-dependent signaling pathway, as it facilitates the cathepsin D-mediated cleavage of caspase-8 and the activation of the transcription factor NF-κB, thus resulting in enhanced cytokine production. Further recruitment of the kinase RIP-1 to the IPS-1-cathepsin D complex initiates the necroptosis of a small number of DCs. The HMGB1 released by the dying cells then enhances the IFN-β production caused by poly IC. Collectively, these findings indicate that cathepsin D-triggered necroptosis is a mechanism that propagates the adjuvant efficacy of poly IC (Figure 2).
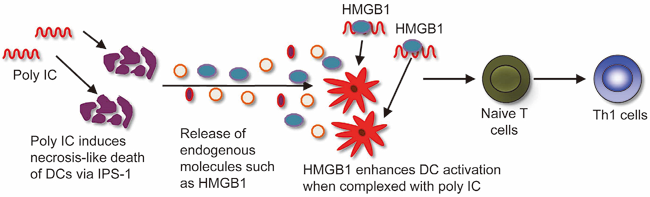
Figure 2. Activation of dendritic cells by poly IC.
3. A novel role of macrophages in the maintenance of adipose tissues
Macrophages consist of at least two subgroups, M1 and M2. Whereas M1 macrophages are pro-inflammatory and play a central role in host defense, M2 macrophages associate with anti-inflammatory reactions and tissue remodeling, among other processes. Genome-wide association studies in humans suggest that TRIB1 participates in lipid metabolism. We found that Trib1 is critical for the differentiation of tissue-resident macrophages, which we term M2-like macrophages (Satoh T et al., Nature, 2013). Trib1-deficiency results in severe reductions of M2-like macrophages in various organs, including adipose tissues. These defects in macrophage differentiation are caused by aberrant expression of C/EBPα in Trib1-deficient bone marrow cells. Mice lacking Trib1 in hematopoietic cells have a diminished adipose tissue mass that is accompanied by evidence of increased lipolysis. In response to a high-fat diet, mice lacking Trib1 in hematopoietic cells develop hypertriglyceridaemia and insulin resistance. Therefore, Trib1 is critical for adipose tissue maintenance and suppression of metabolic disorders by controlling the differentiation of tissue-resident M2-like macrophages (Figure 3).
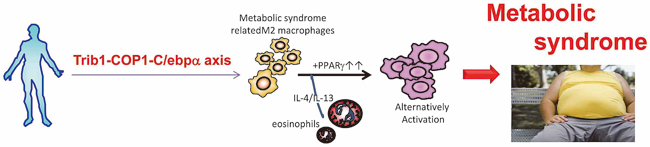
Figure 3. A role of M2-like macrophages.
Our future research will aim to generate a comprehensive understanding of the innate immune system and to develop an effective treatment for immune-related inflammatory diseases.